1. Introduction
Children’s multisystem inflammatory syndrome can appear three to 5 weeks after Coronavirus exposure. In multisystem inflammatory syndrome in children (MIS-C), the condition is confirmed with a positive corona virus disease-2019 (COVID-19) test result for the infant or relatives, a fever that lasts three to four days, and the involvement of two or more organs (e.g., heart, lung, gastrointestinal tract, skin, kidney, joints) (1). It is necessary to increase inflammatory markers and to be ruled out other differential diagnoses. By intensity, we categorize the disease into mild, moderate, and severe conditions. Symptoms may not show up in the mild type, and the inflammatory reaction may not need intervention. The mild symptoms manifest in rare cases without severe multi-organ involvement, including fever, cutaneous rashes, alterations in blood cells, blood ferritin concentration, and high C-reactive protein (CRP) levels (2). In the intermediate type, the disease is controlled. In severe cases with a high fever, the patient must be hospitalized in the intensive care unit and receive higher doses of corticosteroids. Neonatal multisystem inflammatory syndrome (MIS-N) is a multisystem inflammatory syndrome that affects various systems in the body and is confirmed with a positive history of polymerase chain reaction (PCR) test in the mother and/or a history of contact with those who are a vector for COVID-19 infection and a positive serologic test in the neonate (3). Neonatal multisystem inflammatory syndrome is hard to diagnose because fever is less common in neonates, and the similarity of symptoms to sepsis requires new criteria for MIS-N diagnosis. In inflammatory syndromes, especially in delayed phases of COVID cytokine storms, the mortality and morbidity diminish with proper interventions and inhibited cytokine cascade inflammations, and it has a good prognosis after treatment on time. Many reports of dermatologic manifestations of multisystem inflammation in children and adults. But, MIS-N mostly manifests with cardiac demonstration and very few reports of dermatologic issues in neonates. We presented two neonates with mild and intermediate manifestations and dermatologic manifestations who underwent treatment and follow-up for MIS-N. We want to help design new criteria for diagnosing MIS-N by introducing these 2 cases.
2. Case Presentation
2.1. Case I
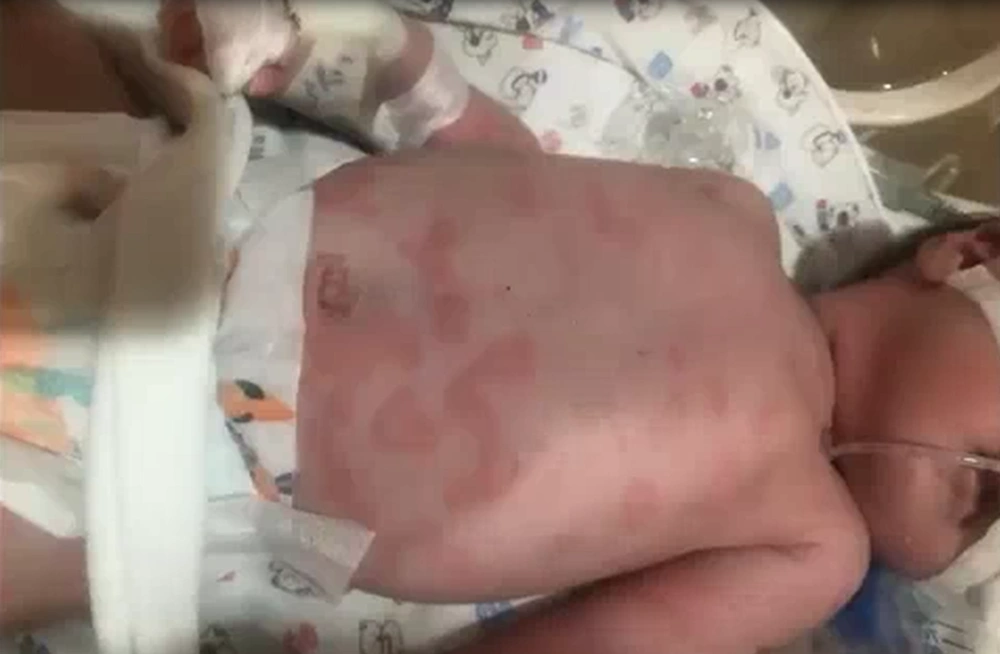
The first case was a male Persian neonate at gestational age (GA): 39 weeks born through the elective cesarean section from a 23-year-old mother with Gravid 1 Live1 and a good APGAR score. It presented his disease on the 17th day after birth with diarrhea, dehydration, and fever for three days, and we admitted him on March 25, 2020 for suspicious neonatal sepsis on his 20th day after birth in the Neonatal Intensive Care Unit of the Children’s Medical Center, Tehran, Iran. Considering the high CRP, we administered antibiotics and treated the dehydration. We performed the COVID-19 PCR test because of the pandemic. The result was negative. The fever was relieved for a period but appeared again. The multi-form macular rash appeared on the trunk and limbs on the fifth day of admission (Figure 1). Dermatology consultation revealed the possibility of a drug reaction or viral infection, with expected spontaneous resolution. No other organs of the neonate were affected. He had no limitations on limb movement and had normal ultrasound examinations. Inflammatory markers and serologic tests for COVID-19 were requested because of high fever recurrence and elevated Fibrinogen, D-Dimer levels, and high COVID IgG: 10. Rheumatologic consultation results brought up the likelihood of MIS-N because of a history of contact with a relative with COVID-19 positive in mother during 35th week of pregnancy and positive on her serology (IgG: 15; IgM: 4). We administered methylprednisolone 30 mg/kg stat intravenous, followed by 1mg/kg oral prednisolone for three days because of patient limitation for staying prolong in the hospital. The neonate became stable after corticosteroid pulse therapy. Fever did not appear during 3 months follow-up after discharge, and the neonate regained his normal condition (Table 1).
| Lab Data | Case I in Admission | Case I During Admission | Case II in Admission | Case II During Admission |
|---|---|---|---|---|
| Blood urea nitrogen (BUN) | 29 | 17 | 6 | - |
| Creatinine (CR) | 0.8 | 0.5 | 0.3 | - |
| Calcium | 9 | - | 9 | - |
| Phosphorous | 4.8 | - | 5 | - |
| Magnesium | 2 | - | 1.9 | - |
| Sodium | 154 | 144 | 137 | - |
| Potassium | 4.6 | 4.2 | 4.5 | - |
| White blood count (WBC) | 23000 | 18000 | 26000 | 7100 |
| Polymorph nuclear leucocytes (PMN) | 15900 | 4200 | 17000 | 1630 |
| Lymphocyte (lymph) | 5500 | 7400 | 3100 | 430 |
| Hemoglobin (HB) | 13.9 | 13 | 12 | 11.9 |
| Platelet (PLT) | 212000 | 212000 | 293000 | 250000 |
| C-reactive protein (CRP) | 93 | 34 | 2 | 1 |
| CSF culture | - | Negative | Negative | - |
| Stool exam | Many fat droplet/ reducing substances: Trace WBC:25-30 RBC:30 | WBC = 8 - 10 | NL | |
| Blood culture | Negative | Negative | - | Negative |
| Urine culture | Negative | Negative | - | Negative |
| COVID PCR | Negative | Negative | - | Negative |
| COVID/serology | - | IgM:2 IgG: 10 | - | IgM:0.1 IgG: 40 |
| Others | D-dimer: 1215 Alanine aminotransferase (ALT):15 Aspartate aminotransferase (AST):30 | D-Dimer: 809 LDH: 453 Ferritin: 553 Fibrinogen: 334CPK: 29 | Alanine aminotransferase (ALT):10 Aspartate aminotransferase (AST):17 | D-dimer: 435 |
| Treatment | Ampicillin amikacin | Vancomycin cefotaxime Rheumatologic consultation: Methylprednisolone cardiologic consultation: Normal function / PFO | Rheumatologic consultation: No. Intervention cardiologic consultation: Normal | Outpatient hydrocortisone |
2.2. Case II
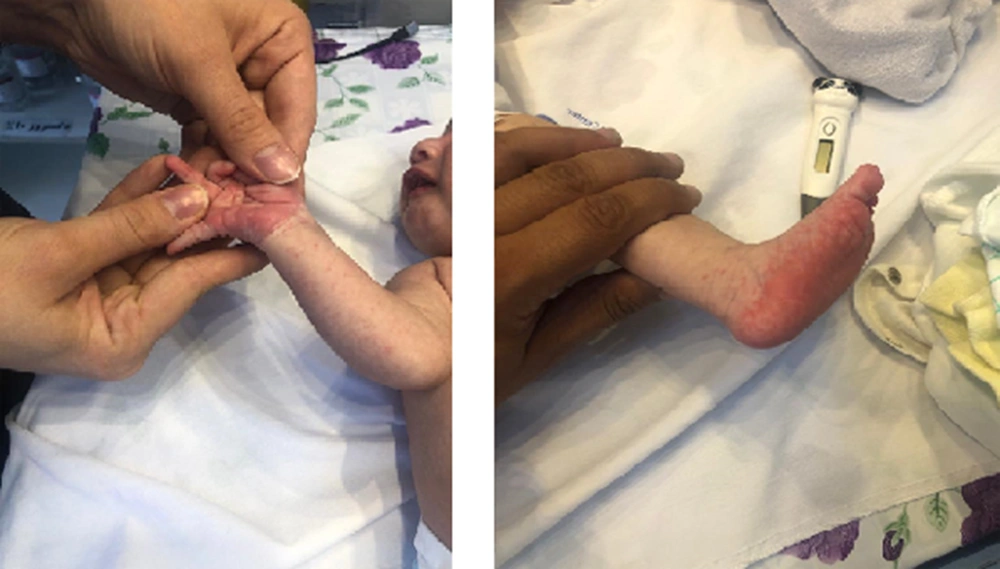
The second case was a cesarean section-born female neonate (GA: 38 weeks). The neonate’s disease presented on her 20th birthday with cough and rashes on the head, palms, and soles for two days (Figure 2), and she was admitted on her 22nd day of birth on September 5, 2020 in the neonatal ward of the Children’s Medical Center, Tehran, Iran. An outpatient center injected a single dose of hydrocortisone with a diagnosis of allergic reactions. The mother was infected with COVID-19 in her 30th week of pregnancy. The physical examination, lab data, and chest X-ray of the neonate were normal except for a high level of COVID-19 antibody and a rash. (IgG: 40; IgM: 0.1). The other organs were intact. The neonate was negative for the COVID-19 PCR test. We could not confirm that there was an infectious disease. The cough and rash disappeared 1 day after a single dose of hydrocortisone. Rheumatologic consultation stated probable mild MIS-N and recommended no aggressive intervention due to receiving a single hydrocortisone dose and alleviating the symptoms. We discharged the case after two days in a stable condition with a recommendation for cardiac and dermatology follow-ups (Table 1). In 3 months of follow-up, the neonate had a normal state.
3. Discussion
Our study presents 2 neonates; that following the manifestation of systemic disease; skin rashes appeared in different parts of the body. The standard treatments did not work for the rash and the systemic disease. We noticed that in both cases, mothers were affected by COVID-19. IgM and IgG titers in the neonates confirmed the passage of antibodies. When steroids were added to the treatment, the symptoms subsided. Neonates’ follow-up was normal after they were discharged.
There are more cases of MIS in children and infants (4) than there are in newborns (5). A study further reported multisystem inflammatory syndrome in fetus (MIS-F) during the fetal period, where the infection starts during the fetal period, and symptoms appear after birth (6), as in the cases of this study.
The serum IgG concentration was 10 g/L in case I and 40 in case II, above normal level 5. The levels could rise over time at subsequent titrations, but we could not check them because we didn’t want to inject the baby again for unnecessary lab data. The serologic test results for 20 neonates were positive in India (3). Since the immune system is immature and non-developed in newborns, positive IgM results are unexpected, though positive maternal IgGs and a history of positive PCR results can be a criterion for detecting MIS-N in newborns (3).
In case II, the mother confirmed infection with COVID-19 on her 30th week of pregnancy. This history is important during the last few weeks of a pregnant woman’s life (3). In cases with no history of maternal infections, mothers must undergo serologic tests to find any clues of infection in newborns, as in case I. In these cases, positive IgM and IgG results are a criterion for MIS-N diagnosis. The neonate’s infections can occur two months after relatives’ infections (7). When there is no evidence of a history of disease and/or positive serologic test results while the infant develops symptoms in the second or third week, infection through carriers who are in contact with the infant can be expected.
The diagnosis criteria for MIS-C are the same in all studies. MIS-C is diagnosed with positive PCR test results and/or a history of contact with those infected or carrying infections and positive serologic test results.
Various case series show the involvement of multiple systems (e.g., skin involvements), spanning children from multi-form to maculopapular rashes and severe lesions such as allergic lesions, urticaria, and even gangrene infection (4). In both cases, the lesions were mild and disappeared after corticosteroid treatment. There are some criteria for MIS in children (8). We can’t use MIS-C criteria for neonates as fever is less common than in children, and neonates have mild symptoms. Exposure to COVID-19 and systemic symptoms with increased inflammatory lab data is enough for neonates.
The Coronavirus disease’s active phase does not include MIS-C. Symptoms of an inflamed system appear two to three weeks after a cytokine storm (9). The first-line treatment, in this case, is corticosteroid pulse therapy (e.g., using methylprednisolone). This technique has been successful in older babies (8) and, thereby, can treat newborns. In case I, we did not start treatment because of alleviated fever and CRP concentrations, but inflammation was not controlled given the fever recurrence and increased CRP levels. Corticosteroid pulse therapy was used to suppress inflammation. The advantages of corticosteroid pulse therapy are diminished hospitalization period, rapid inflammatory reaction alleviation in the inflammatory phase, and no need for the simultaneous use of non-steroidal anti-inflammatory drugs. In this condition, methylprednisolone is a potent medication for suppressing inflammation (10).
In many places, intravenous immunoglobulin therapy (IVIG) (11), used for cardiogenic shocks in patients infected with COVID, is not well accepted because of high expenses, limited availability, and fluid restriction during shocks. The Iranian national guideline recommends the implementation of IVIG in cases when corticosteroids don’t work, and vital organs or coronaries are affected. The standard dose of methylprednisolone in children is 10 to 30 mg/kg as a 2- to -3-hour infusion for three days (and five days in severe cases) (8). In case I, 30 mg/kg was administered for one day and followed by 1 mg/kg of oral prednisolone for three days because of the patient’s limited stay in the hospital.
Studies have reported up to 90% cardiac involvement, myocardial involvement as a cardiac block, or even cardiogenic shock in patients with MIS-C. For this, both cases underwent echocardiography. With cardiac involvement, proper interventions and regular cardiac follow-ups are required, especially at lower ages (12), establishing at least two follow-up sessions and checking inflammatory markers within two weeks to two months (3).
In case I, the neonate had a high D-dimer level but decreased in subsequent tests without anticoagulation medication. Anticoagulants, such as enoxaparin (in urgent cases for patients admitted to the intensive care unit) and aspirin (dosage: 3 - 5 mg per kg of weight, for less severe conditions and patients not admitted to the intensive care unit), have been advised for COVID infection with a high risk of thrombosis and coagulopathy. Such medications are administered depending on the infant’s condition because the risk of thrombosis is low at lower ages and newborns (3).
Persistent pulmonary hypertension (PPHN) can be related to MIS-N, but we didn’t find it. Dexamethasone is used to alleviate persistent pulmonary hypertension in neonates, which is reported by McCarty et al. (6). Some studies used the DART (dexamethasone: A randomized trial) protocol for dexamethasone (13). Dexamethasone varies from methylprednisolone in the drug’s efficacy. The former is a long-action drug, and the latter a short-action medication. High doses of methylprednisolone suppress and restart the immune system by rapid and short-term operating, while dexamethasone’s half-life in blood is longer and can cause long-term immune system suppression implications (14).
3.1. Limitations and Recommendations
Neonatal multisystem inflammatory syndrome is a term that was added to neonatal literature recently. A delay follows the issue in diagnosis. Suggested criteria for MIS-N are applicable when we think about it. Besides, many suggested inflammatory markers, are not checked routinely in neonates as Ferritin. We believe it may be better to revise or define the suggested markers as a key for diagnosing the titer of COVID-19 antibodies.
3.2. Conclusions
For the similarity of symptoms to sepsis, we should consider this syndrome in infants with sepsis-like symptoms and a positive history of COVID in parents or relatives. Neonatal multisystem inflammatory syndrome does not appear to fill the MIS-C criteria as they do in children with sepsis symptoms. We suggest that combination criteria such as exposure to COVID-19 and systemic symptoms with increased inflammatory lab data are enough for neonates, but more studies are needed. If we consider proper and on-time interventions, it is possible to diminish morbidity and mortality caused by inflammation. Additional trials are required for the use of corticosteroids in newborns.