1. Background
In parallel with the augmenting incidence of obesity worldwide, non-alcoholic fatty liver disease (NAFLD) is increasingly recognized as one of the leading causes of chronic liver disease (1). NAFLD was first described in 1980 by Ludwig et al. in subjects with no history of alcohol consumption (2). This disease includes simple steatosis and non-alcoholic steatohepatitis (NASH), which lead to fibrosis and, eventually, cirrhosis. Steatosis is almost always observed in morbidly obese subjects, and the prevalence of NASH in these people is estimated to be 25 - 70% (3, 4). NAFLD is frequently associated with obesity, type 2 diabetes mellitus, hyperlipidemia, and insulin resistance, which are the main features of metabolic syndrome (5). NAFLD is also attributed to an increased systemic proinflammatory state that accelerates atherosclerosis and thereby raises the risk of associated cardiovascular disorders (6). Among the underlying factors of NAFLD, obesity and type 2 diabetes can be mentioned, and these two diseases are also related to insulin resistance and glucose intolerance (7). With the increasing prevalence of obesity, the rate of NAFLD has augmented in children and adults (8). The reported estimates of NAFLD prevalence in obese children vary from 1.7 to 85% in different investigations, with no consensus on this subject (9, 10). In the near future, fatty liver disease prevalence is expected to increase (11, 12). In addition to the specific adverse health outcomes of obesity, including NAFLD, obese boys may suffer from a small penis, overactive bladder, poor body image, decreased quality of life, anxiety, and reduced physical activity. It seems that these issues affect the nutrition status of children and increase the risk of NAFLD in obese children (13, 14). A meta-analysis in China in 2015 stated the prevalence of NAFLD in obese children and adolescents as 34.2%. It was also reported that the prevalence of NAFLD was higher in men compared to women, and the incidence rate increased with higher BMI (15). There is obvious evidence to suggest the significance of sex hormones in the occurrence of NAFLD. Several surveys suggest that estrogens are a protective factor for the development of NAFLD (16). The growth and development of children are very fast, and their pathophysiological changes are very different from adults. Currently, obese children are exposed to maternal obesity and insulin resistance earlier than decades ago (17). Timely diagnosis and rapid intervention for high-risk obese pediatrics can reduce and delay the clinical course. Therefore, it is very momentous to evaluate obese children and search for a sensitive index to predict early liver damage and associated dyslipidemia (18).
Considering industrial development in Iran and the changing lifestyle of individuals, obesity is increasing, especially in children. Recently, due to the rise in obesity in the community, the incidence of NAFLD has augmented. Improper lifestyle and diet put children at risk of fatty liver. The importance of this disease is due to the destruction of liver cells. If not diagnosed in time and not treated properly, it can lead to irreversible liver cirrhosis (19).
2. Objectives
Many studies have been conducted in the field of NAFLD in children. In these similar investigations, the diagnosis of fatty liver was based on liver ultrasonography. However, according to the latest guidelines, screening is based on ALT, and ultrasound is not a screening tool for fatty liver alone. Our research applied a different methodology, and the actual prevalence of NAFLD in children is largely unknown. Consequently, we decided to have ultrasound along with ALT as a screening tool for fatty liver screening in order to investigate the prevalence of NAFLD and related factors in Tehran schools.
3. Methods
In this cross-sectional study, overweight and obese students (BMI ≥ 25) from Tehran schools aged 7 - 17 years were included. After obtaining informed consent from the parents, 115 students were selected and referred to the Gastroenterology, Liver, and Children's Nutrition Research Center of Mofid Hospital for ALT enzyme measurement and ultrasonography. The participants were selected by convenience sampling method from schoolchildren. The sample size was calculated using the formula
Students aged 7 - 17 years with overweight or obesity (BMI ≥ 25) were enrolled in our study. Children with other causes of obesity (e.g., Cushing's disease and hypothyroidism), chronic drug use, history of alcohol addiction, and hepatitis caused by other causes (e.g., viruses and hereditary diseases) were excluded. Patients with high ALT were tested again three months later; if ALT was high, they were considered to have fatty liver. In fact, the diagnosis of fatty liver was based on abnormal ultrasound or ALT higher than twice the ULN during the last three months. Overweight and obese subjects were divided into two groups with fatty liver and without fatty liver. Liver ultrasonography was performed for the sonographic evidence of fatty liver disease. Ultrasonography is the most prevalent technique used to evaluate the presence of fatty liver in clinical settings. Fatty liver is diagnosed according to the following ultrasound parameters: A diffuse hyper-echoic texture (bright liver) as mild, increased liver echo texture compared to the kidneys as moderate, and vascular blurring and deep attenuation as severe (21). Demographic data (e.g., age and gender), physical examination findings (e.g., blood pressure, hepatomegaly, splenomegaly, jaundice, itching, fat accumulation in the shoulder, and symptoms of hypothyroidism), family history (e.g., diabetes, obesity, fatty liver, and dyslipidemia), level of physical activity, smoking, nutritional status, anthropometric indicators, and liver ultrasound results were recorded in the designed questionnaire. Laboratory measurements were performed, including fasting blood sugar (FBS), serum lipid profile, and liver enzymes. Next, these two groups were compared regarding demographic and anthropometric characteristics.
3.1. Statistical Analysis
SPSS version 22 software was applied for statistical analysis. Mean ± SD and percentage were used to describe the quantitative and qualitative variables, respectively. In order to test distribution, Kolmogorov-Smirnov and Shapiro-Wilk tests were utilized. Differences were compared using the chi-square test. Moreover, the Hosmer-Lemeshow test was used to fit logistic regression models. P < 0.05 was considered significant.
4. Results
The mean age of participants was 12.6±4.8 years old. In this study population, the prevalence of fatty liver was 47.8%, with 15 (27.3%) girls and 40 (72.7%) boys being affected. The two genders had a statistically significant difference (P = 0.02). Moreover, BMI was significantly higher in children with fatty liver. Gender was significantly different in two groups with and without fatty liver.
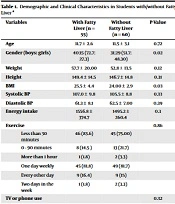
Obesity and a parent's history of diabetes and fatty liver were not recognized as risk factors for fatty liver in offspring. Clinical symptoms for each of them were uniform, and no appreciable variations were found. Jaundice, hepatomegaly, xanthoma, and slippage of femoral epiphysis were not found in any of the students in either group. The percentage of students with fat accumulation was 16.5% overall, 14.5% in those with fatty livers, and 18.3% in those without fatty liver. Fat accumulation was not remarkably different between the two groups. Demographic and clinical characteristics are provided in Table 1.
| Variables | With Fatty Liver (n = 55) | Without Fatty Liver (n = 60) | P-Value |
|---|---|---|---|
| Age | 11.7 ± 2.6 | 11.5 ± 3.1 | 0.72 |
| Gender (Boys: Girls) | 40:15 (72.7: 27.3) | 31:29 (51.7: 48.30) | 0.02 |
| Weight | 57.7 ± 20.00 | 52.8 ± 13.5 | 0.12 |
| Height | 149.4 ± 14.5 | 146.7 ± 14.8 | 0.31 |
| BMI | 25.5 ± 4.4 | 24.00 ± 2.9 | 0.03 |
| Systolic BP | 107.0 ± 9.8 | 105.5 ± 8.8 | 0.33 |
| Diastolic BP | 61.3 ± 8.1 | 62.5 ± 7.00 | 0.39 |
| Energy intake | 1556.8 ± 374.7 | 1495.2 ± 260.4 | 0.3 |
| Exercise | 0.86 | ||
| Less than 30 minutes | 46 (83.6) | 45 (75.00) | |
| 0 - 90 minutes | 8 (14.5 ) | 13 (21.7) | |
| More than 1 hour | 1 (1.8) | 2 (3.3) | |
| One day weekly | 45 (81.8) | 49 (81.7) | |
| Every other day | 9 (16.4) | 9 (15) | |
| Two days in the week | 1 (1.8) | 2 (3.3) | |
| TV or phone use | 0.32 | ||
| Less than 2 hours | 44 (80.00) | 49 (81.7) | |
| More than 2 hours | 11 (20) | 11 (18.3) | |
| History of DM in parents | 0.33 | ||
| Mother/father | 38 | 34 | |
| None | 15 | 25 | |
| Both | 2 | 1 | |
| History of DL in parents | 0.54 | ||
| Mother/father | 36 | 38 | |
| None | 4 | 8 | |
| Both | 15 | 14 | |
| History of FL in parents | 0.26 | ||
| Mother/father | 25 | 21 | |
| None | 17 | 19 | |
| Both | 0 | 2 | |
| Others | 17 | 19 | |
| History of obesity in parents | 0.64 | ||
| Mother/father | 38 | 45 | |
| None | 12 | 10 | |
| Both | 5 | 4 | |
| Others | 0 | 1 | |
| Clinical symptoms | |||
| Spleenomegaly | 12 (21.8) | 9 (15.0) | 0.34 |
| Itching | 2 (3.6) | 2 (3.3) | 0.92 |
| Acanthosis | 3 (5.5) | 3 (5.0) | 0.91 |
| Stretch marks | 14 (25.5) | 13 (21.7) | 0.63 |
| Fat accumulation | 8 | 11 | 0.58 |
Abbreviations: DM, diabetic mellitus; FL, fatty liver; DL, dyslipidemia; BP, blood pressure.
a Values are expressed as No. (%) or mean ± SD.
The two groups had no significant difference in FBS and triglyceride (P > 0.05). AST and ALT were significantly different between the children with and without NAFLD (P = 0.002, P < 0.001, respectively). The levels of AST and ALT in patients with NAFLD were higher than in subjects without NAFLD. Total cholesterol was higher in children with NAFLD than in the other group (P = 0.006). Blood biochemistry markers are shown in detail in Table 2.
| Variables and Measurement | With Fatty Liver (n = 55) | Without Fatty Liver (n = 60) | P-Value |
|---|---|---|---|
| FBS | 95.3 ± 7.1 | 95.0 ± 9.2 | 0.86 |
| Normal | 55 (100) | 60 (100) | |
| Abnormal | - | - | |
| TG | 138.3 ± 46.3 | 135.0 ± 38.1 | 0.98 |
| Normal | 32 (58.2) | 35 (58.3) | |
| Abnormal | 23 (41.8) | 25 (41.7) | |
| AST | |||
| First visit | 29.0 ± 8.0 | 25.2 ± 4.9 | 0.002 |
| Normal | 36 (65.5) | 53 (88.3) | |
| Abnormal | 7 (11.7) | 18 (15.7) | |
| After 3 months | 32.1 ± 5.4 | - | |
| ALT | |||
| First visit | 31.7 ± 16.3 | 19.9 ± 5.2 | < 0.001 |
| Normal | 47 (85.5) | 60 (100) | |
| Abnormal | 8 (14.5) | - | |
| After 3 months | 38.0 ± 10.7 | - | |
| T Chol | 176.6 ± 25.8 | 168.4 ± 25.6 | 0.006 |
| Normal | 41 (74.5) | 4 (6.7) | |
| Abnormal | 14 (25.5) | 4 (6.7) | |
| Alb | 4.4 ± 0.47 | 4.4 ± 0.51 | 0.91 |
| PT | 28.4 ± 1.7 | 28.3 ± 1.6 | 0.82 |
| INR | 1.0 ± 0.01 | 1.0 ± 0.01 | 0.95 |
Abbreviations: FBS, fasting blood sugar; TG, triglyceride; AST, aspartate transaminase, ALT, alanine transaminase; T Chol, total cholesterol; Alb, albumin; PT, prothrombin time.
a Values are expressed as No. (%) or mean ± SD.
According to the results of the univariate logistic regression, the prevalence of fatty liver significantly correlated with gender, BMI, total cholesterol, AST, and ALT. The findings clearly indicate the higher vulnerability of boys than girls, and the female gender reduced the risk of the disease (OR = 2.45, 95% CI: 1.14 - 5.44). In addition, the risk of fatty liver would rise by 1.12 times with each unit increase in BMI (95% CI: 1.00 - 1.26, P = 0.03). The overall prevalence of fatty liver was higher in students with abnormal cholesterol. The odds of developing fatty liver were 4.78 (95% CI: 1.46 - 15.58, P = 0.009) times greater than those of students with normal cholesterol.
In students with abnormal AST levels compared to students with normal AST levels, the chance of having fatty liver increased 3.99 times (95% CI: 1.52 - 10.48, P = 0.005). Elevating 1 U/L ALT increased the likelihood of having fatty liver by 18% (95% CI: 1.09 - 1.28, P < 0.001). The onset of fatty liver was unrelated to age, calorie intake, alcohol intake, systolic and diastolic blood pressure, FBS, triglyceride, history of illnesses, and other laboratory variables. Exercise protected against the likelihood of getting fatty liver; however, this benefit was not statistically significant.
We used the backward approach in the multivariate analysis, and the variables with P-value < 0.25 in the univariate analysis were included in the multivariate model. The results of the modified model indicated that total cholesterol and ALT were significant contributors to the onset of fatty liver. The Hosmer-Lemeshow test and the area under the receiver operating characteristic (ROC) curve were applied to evaluate the outcomes of the logistic regression model in terms of model fit. However, the area under the ROC curve was evaluated at 0.81 (CI: 0.73 - 0.89), and the Hosmer-Lemeshow test did not show statistical significance (P = 0.58). More details about univariate and multivariate logistic regression are shown in Table 3.
| Variables | Odds Ratio (95% Confidence Interval) | P-Value |
|---|---|---|
| Univariate Analysis | ||
| Boys (Reference: Girls) | 2.45 (1.14 – 5.44) | 0.02 |
| Age | 1.02 (0.09 – 1.16) | 0.72 |
| BMI | 1.12 (1.00 – 1.26) | 0.03 |
| Systolic blood pressure | 1.02 (0.98 – 1.06) | 0.33 |
| Diastolic blood pressure | 0.97 (0.93 – 1.02) | 0.39 |
| Energy intake | 1.00 (0.99 – 1.00) | 0.3 |
| Exercise; 0 - 90 minutes (reference: < 30 minutes) | 0.6 (0.22 – 1.59) | 0.3 |
| More than 1 hour | 0.48 (0.04 – 2.58) | 0.56 |
| Less than once a week (Reference: Never) | 2.22 (0.19 – 7.42) | 0.51 |
| FBS | 1.00 (0.96 – 1.04) | 0.86 |
| TG | 1.00 (0.99 – 1.01) | 0.66 |
| T Chol (Reference: Normal) | 4.78 (1.46 – 15.58) | 0.009 |
| AST (Reference: Normal) | 3.99 (1.52 – 10.48) | 0.005 |
| ALT (Reference: Normal) | 1.18 (1.09 – 1.28) | < 0.001 |
| Multivariate Analysis | ||
| Boys (Reference: Girls) | 1.55 (0.61 – 3.89) | 0.35 |
| BMI | 1.10 (0.97 – 1.25) | 0.12 |
| T Chol (Reference: Normal) | 3.65 (1.00 – 13.92) | 0.04 |
| AST (Reference: Normal) | 1.06 (0.27 – 4.16) | 0.92 |
| ALT (Reference: Normal) | 1.16 (1.06 – 1.27) | 0.001 |
Abbreviations: FBS, fast blood sugar; TG, triglyceride; AST, aspartate transaminase, ALT, alanine transaminase; T Chol, total cholesterol.
5. Discussion
NAFLD has become the most prevalent reason for chronic liver disease in pediatrics, paralleling the increasing prevalence of obesity worldwide. The current study aimed to investigate the prevalence of fatty liver and its related factors in overweight and obese students in schools in Tehran. In our study, the overall prevalence of NAFLD in students was 47.8%. In summary, in the present study, the levels of AST and ALT in patients with NAFLD were higher than in subjects without NAFLD, and the prevalence of fatty liver significantly correlated with gender, BMI, total cholesterol, AST, and ALT. Based on our findings, the risk of fatty liver would increase by 1.12 times with each unit increase in BMI. The overall prevalence of fatty liver in Adibi et al. and Shiasi Arani et al. studies was 54.4 and 53.3%, respectively (22, 23), which is similar to the result of the present study.
Pawar et al., in a school-based cross-sectional study in Mumbai, reported the prevalence of NAFLD in overweight and obese children to be 66.1%, which is slightly higher than our study (24). In the research by Atwa et al., the prevalence of fatty liver was 38.90%. A mild degree of fatty liver infiltration was observed in most of the subjects (25). However, it is difficult to compare the prevalence between different populations because published data differ in terms of study design, sample selection, a diagnostic method used to define fatty liver, as well as the age, gender, and ethnicity of the study populations. These cases may be the cause of differences in prevalence between various studies.
Alavian et al. stated that it seems the main reason for the controversial results of fatty liver prevalence is using different techniques for diagnosing fatty liver. For example, the prevalence reported using ultrasound was higher than liver enzymes (26). In our study, 72.7% of boys and 27.3% of girls had fatty liver, and a significant difference was observed between genders (P = 0.02). In an Indian study by Agrawal and Duseja among 1168 patients diagnosed with ultrasound, the prevalence of NAFLD in men was 16.6% higher than in women (27). Furthermore, Schwimmer reported that fatty liver was significantly more common in boys than in girls (28), which is in line with the results of the current study. The higher prevalence in boys results from excess body fat distributed in the abdominal area and the effect of sex hormones.
In the investigation by Kelishadi et al., the prevalence of fatty liver in the two genders did not show a significant difference, which is contrary to the findings of the present study. This lack of difference may be due to the younger mean age in the mentioned study compared to our research, which can be justified due to the absence of sex hormones at a young age and their effects (29). In the present study, the prevalence of fatty liver was 21.8%, 61.8%, and 16.4% in the age groups of 7 - 10, 10 - 14, and 14 - 17 years, respectively. The findings of Shiasi Arani et al. showed that the increase in the prevalence of fatty liver is related to older age in children and adolescents (23), showing the importance of paying more attention to diagnosing and treating fatty liver in obese adolescents compared to obese children.
Atwa et al. reported that BMI and waist circumference (WC) were significantly higher in the NAFLD group and stated that increased BMI is a strong predictor of NAFLD (25). Moreover, according to Hagstrom et al., having a high BMI in adolescence raises the chance of developing a critical liver disease by 5% for every 1 kg/m2 increase in BMI (30). Damaso et al. demonstrated that for each centimeter rises in WC, there was a two-fold increase in the risk of developing NAFLD in obese and overweighed children (31).
Fatty liver is one of the disorders related to metabolic syndrome, and obese people are more likely to be affected by metabolic syndrome in adulthood than other people (29). Similar to our findings, Atwa et al. showed that total cholesterol level had a significant relationship with NAFLD, and the total cholesterol level was higher in children with NAFLD than in healthy ones (25). Contrary to our results, Gupta et al. demonstrated that lipid levels in children with and without NAFLD were not different (32). Based on the findings of the present study, the increase of AST and ALT is associated with fatty liver, and we found that 34.5% of patients with fatty liver and 11.7% of people without fatty liver had abnormal AST levels. The average ALT is higher in patients with fatty liver than in healthy subjects, and 14.5% of people with fatty liver had abnormal ALT levels. The latter difference was significant between the two groups (P < 0.05). In addition, the univariate logistic regression model results to measure factors related to fatty liver in students showed that fatty liver had significant positive correlations with AST and ALT. A multicenter study in India stated high serum ALT and AST levels as risk factors for NAFLD. In patients with fatty liver, ALT and AST levels were higher than those without fatty liver (27), which is consistent with the findings of the present research. In the study of Elizondo-Montemayor et al. on 236 children aged 6 - 12 years, 17.7% of the obese and overweight population had ALT higher than the normal level. Furthermore, ALT and AST strongly correlated with metabolic syndrome and fatty liver disease (33). The mentioned findings are similar to the result of the current study. Considering this finding and the fact that NAFLD has a direct relationship with metabolic syndrome, it is possible to use serum ALT level as an important marker in the diagnosis, especially for prevention at a young age. In the study of Shiasi Arani et al., high ALT levels were not necessarily associated with fatty liver (23), which is contrary to the results of the present study. This difference may be attributed to the different age ranges of the studied groups. Yoo et al. indicated a higher aminotransferase level in patients with fatty liver than in healthy people (34). The results of Alavian et al. showed that among metabolic variables, ALT increase was the most important predictor of NAFLD and was related to the progression of NAFLD (26). According to the report of Das et al., abnormal liver enzymes in overweight children were considerably higher than normal weight children (20).
The results of our study are also consistent with previous studies that evaluated the diagnostic accuracy of ALT for NAFLD in obese children (32). Although different definitions of abnormal liver enzymes and various diagnostic criteria have been used in various investigations, individuals with NAFLD still had significantly higher ALT compared to children without NAFLD in most studies.
One of the limitations of this study was the small sample size, and due to the COVID-19 pandemic, the closure of schools, and limited time, it was not possible to have a larger sample size. Moreover, the non-availability of a confirmatory test, such as a liver biopsy, is a limitation of our study.
One of the effective sources for this age group is social media, and many habits and lifestyles are transferred to this group through the media. Therefore, social media should have programs to improve lifestyle and encourage children and teenagers to have a normal weight. Early diagnosis using various simple screening tools, such as the measurement of ALT level, can help prevent chronic liver disease and related diseases in obese children. It is recommended to regularly measure weight and WC as indicators of obesity in this age group. Urgent public health measures are required to prevent and control NAFLD in Iranian schoolchildren, and screening of overweight children (BMI > 25) is recommended in terms of NAFLD.
5.1. Conclusions
In our study, BMI was significantly higher in children with fatty liver than in subjects without fatty liver. Based on our findings, the prevalence of fatty liver was significantly correlated with male gender, BMI, total cholesterol, AST, and ALT. A high BMI is associated with the progression of liver disorders toward fibrosis and cirrhosis. Therefore, weight loss and obesity control can be health priorities.