1. Introduction
Chronic granulomatous disease (CGD) is a rare hereditary immunodeficiency disorder characterized by phagocytic defects associated with an abnormal neutrophil oxidative burst. It can appear at any age, from infancy to adulthood, but the mean age of patients diagnosed is 1 to 3 years old. The different genetic mutations of CGD are in the gp91phox, p47phox, p22phox, p67phox, and p40phox genes. The intracellular killing function of the patient’s phagocytic cells was defective, but other functions of phagocytic cells, B cells, and T cells remained normal (1, 2).
The chronic granulomatous disease usually presents with recurrent deep abscesses or granulomas and involves multiple systems, such as the lungs, skin, lymph nodes, liver, spleen, and bone. The patient’s response to infection with fever and an appropriate local inflammatory response leads to granulomatous lesions characterized by assemblages of phagocytic and giant cells containing pigmented lipid material. Subcutaneous abscesses, skin furunculosis, eczematoid dermatitis, impetigo, recurrent pneumonia, hilar lymphadenopathy, empyema, and lung abscess are the hallmark and complications of CGD (3, 4).
Recurrent infections and inflammatory complications caused by bacterial or fungi pathogens, such as Staphylococcus aureus, Burkholderia cepacia, Serratia marcescens, Nocardia spp., Aspergillus spp., Salmonella, Bacillus Calmette-Guérin (BCG), and tuberculosis (TB) in CGD, are common. Mucormycosis infection is an unusual form of fungal infection in CGD and is often present in patients with impaired cell-mediated immunity, such as hematologic malignancies, steroid therapy, and diabetes (1, 5).
Measurement of superoxide production, ferricytochrome c reduction, chemiluminescence, nitroblue tetrazolium (NBT) reduction, or dihydrorhodamine (DHR) oxidation can aid in the diagnosis of CGD (2, 3, 6, 7).
The primary treatment goal for patients with CGD is to prevent and heal infected areas and lesions. Long-term use of trimethoprim-sulfamethoxazole (TMP-SMZ) for prophylaxis reduces recurrent infections. Recent studies have shown that fungal infections are common and the leading cause of mortality in CGD. Antifungal treatment with oral amphotericin B and active azole antifungals, such as itraconazole, voriconazole, and posaconazole, has diminished mortality. Hematopoietic stem cell transplantation (HSCT) is the only curative treatment in these patients (1, 4, 6, 8).
Kakeya indicated that azole-resistant infection with mucormycosis was reported in patients receiving long-term prophylaxis with itraconazole. Mucormycosis culture usually does not yield growth results, and histopathological identification with a typical Mucorales structure may provide the only evidence of infection. Miladinovic et al. declared that the histopathological findings of cutaneous granulomas might be similar to those of Crohn disease, sarcoidosis, and TB. Occasionally an additional pathogen is identified in the patient’s skin specimen, and eosinophils are not abundant. Therefore, a review of previous studies reveals that physicians have limitations in diagnosing and treating CGD patients (3, 9). In this case report, we discussed a 23-month-old infant with lymphadenopathy recently diagnosed with CGD and lung involvement while receiving prophylactic treatment. The patient’s radiography showed a mass-like consolidation in her right lung, and she was infected with mucormycosis.
2. Case Presentation
A 23-month-old female infant was admitted to our pediatric center (Gorgan, Iran) with a chief complaint of lymphadenopathy on the right side of the neck. The lymph node developed gradually over a month, and during that time, she had no symptoms of fever, chills, nausea, vomiting, weight loss, or sweating.
There were no complications during pregnancy and delivery and no particular family history. Her birth weight was 3200 g, her height was 39 cm, and her head circumference was 26.5 cm. Her current weight is 9000 g, and her height is 84 cm. All her growth parameters were below the 5th percentile at birth and for the past 23 months. She was hospitalized four months ago due to left lung empyema for 14 days and discharged in good condition with proper treatment.
On physical examination, the patient’s vital signs were stable, and a cervical lymph node approximately 1.5 × 0.5 cm in size with significant tenderness was found in the right submandibular region. A tender, mobile lymph node with no cutaneous changes, such as erythema or warmth, was found on palpation. No other mass was found. In addition, no skin lesions and scars on the neck, hoarse voice, shortness of breath, and wheezing were found. The normal heart (S1 and S2) and lung sounds were auscultated. The abdominopelvic examination was normal, and splenomegaly and hepatomegaly were not found.
During the admission, laboratory tests, chest X-rays, and sonography of lymphadenopathy were requested. On laboratory tests, the hemogram revealed anemia and leukocytosis with lymph dominance. The baseline C-reactive protein (CRP) concentration increased (9.1 mg/dL), and the patient’s erythrocyte sedimentation rate (ESR) was 40 mm/s. Other laboratory tests and chest X-rays were normal. Due to the prevalence of TB in our region, we considered a purified protein derivative (PPD) test for TB, which was negative. In addition, because of the COVID-19 pandemic, a polymerase chain reaction (PCR) test was done, which was negative, too.
On ultrasound, the right cervical lymph node measured 17 × 8.5 mm, with significant tenderness was seen. Also, abdominal and pelvic sonography was normal, and three times of gastric washing specimens for TB were negative. A biopsy was performed for diagnosis, and chronic granulomatous inflammation with foci of necrosis was reported. The neutrophil oxidative burst assay was 90%, and the NBT test was 6%, which were both highly positive for CGD. After performing a biopsy, the patient was diagnosed with an autosomal recessive CGD and discharged in good condition with proper treatment.
Unfortunately, two months later, the patient returned with a complaint of fever, cough, nausea, and vomiting, which were not responded to outpatient treatment with no further complaints. Her physical examination detected only fever and coarse crackles in both lungs. Her laboratory tests showed anemia and leukocytosis with high CRP and ESR. Because of the patient’s fever of unknown cause, blood and fungal cultures and TB tests were ordered, but the results were negative. The interferon-gamma release assay (TB-IGRA) was negative as well.
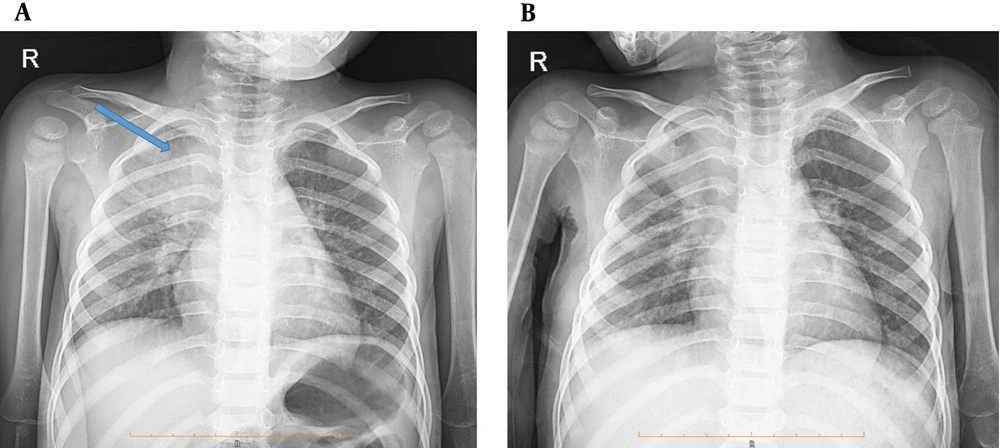
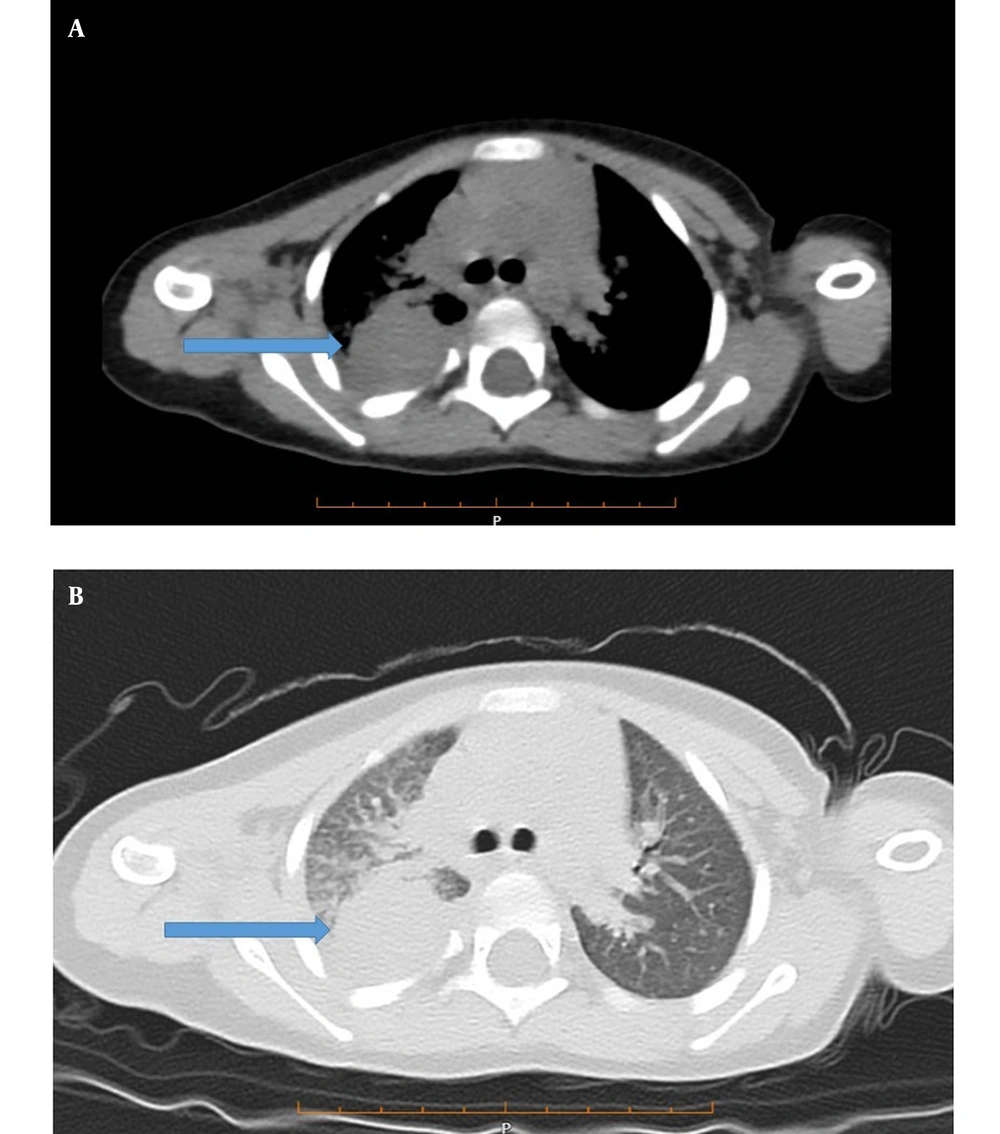
According to the patient’s chest X-ray, a homogeneous opacity was observed in the right lung (Figure 1A). A computed tomography (CT) scan of the lung showed a 30-mm right upper lobe mass-like consolidation and interstitial opacities. Patchy consolidation in both peripheral areas of the lungs, especially in the middle and lower lobes, linear atelectasis in the lower lobes, and right pleural effusion (approximately 100 mL) were also observed (Figures 2A and 2B).
Based on her clinical features and paraclinical results, antibiotic therapy started. Bronchoscopy was done, and bronchoalveolar lavage (BAL) sample was taken. Necrotizing granulomatous fungal inflammation compatible with mucormycosis was observed in the result of the BAL sample. A pediatric specialist surgeon performed a biopsy from mass-like consolidation. Multiple granulomatous with necrosis, mixed inflammatory cells and a layer of fungal hypha were reported in a periodic acid-Schiff (PAS) staining test. The Ziehl–Neelsen (ZN) test for TB detection was negative. Hence, the pathology report confirmed the BAL result, which was a mucormycosis infection. For further investigation of systemic mucormycosis infection and unspecific gastrointestinal symptoms in the patient, an abdominopelvic CT scan with contrast was performed, which was normal. Therefore, standard antifungal treatment with amphotericin B was started, and the patient’s fever and symptoms improved. The patient was discharged two weeks later with antifungal treatment with oral voriconazole and trimethoprim/sulfamethoxazole. Also, she was advised and referred to the pediatric center for follow-up treatment and to do HSCT (Figure 1B).
3. Discussion
We present a 23-month-old infant with right lymphadenopathy recently diagnosed with CGD. Two months later, our patient presented uncontrolled fever and a mass-like consolidation during her radiological evaluation while receiving trimethoprim/sulfamethoxazole prophylaxis. The pulmonary mucormycosis infection was confirmed based on the biopsy and BAL pathology reports. In our case report, even though our patient did not receive any immunosuppressive medication nor had a history of hematologic malignancies or diabetes, she was infected with mucormycosis, which was presented with an infrequent feature as a mass-like consolidation. After initiation of amphotericin B, her symptoms improved, and she was discharged in good general condition. After that, she was consulted and referred for HSCT.
The chronic granulomatous disease is a rare, life-threatening primary immunodeficiency disease, mostly inherited in an X-linked or autosomal recessive disorder. Patients usually develop symptoms through the second and third years of life. The recurrent infection of pneumonia, abscess, lymphadenopathy, suppurative adenitis, osteomyelitis, bacteremia/fungemia, cellulitis, and meningitis is common in CGD patients. Consistent with our case, our patient was diagnosed at 23 months of age and had a history of hospitalization for pneumonia and empyema (1, 3, 4).
The inability to generate superoxide in patients with CGD results in an inability to induce the charge changes required for the fusion of granular components with primary phagocytosis. This defect manifests in defective microbial destruction and recurrent infections with bacteria and fungi. Functional and biochemical analysis of the CGD defect has yielded an impressive amount of information on the role of oxidative and non-oxidative mechanisms in bacterial and fungal killing. The diagnosis of CGD is based on the evaluation of reactive oxygen species (ROS) production by neutrophils. For this purpose, we use a flow cytometric dihydrorhodamine assay and a histochemical NBT test. To diagnose CGD, NBT is sensitive and specific. Our patient had a chief complaint of lymphadenopathy that progressed in a month with no constitutional symptoms. Chronic granulomatous inflammation with foci of necrosis was reported according to the excisional biopsy result. Then, based on the positive results of the neutrophil oxidative burst assay and NBT test, the diagnosis of CGD was confirmed for her (3, 5, 6).
Patients with CGD are susceptible to various bacterial, fungal, and yeast infections throughout their lives. Nemours bacterial and fungal pathogens commonly threaten these patients. Staphylococcus aureus, Burkholderia spp., Serratia marcescens, Nocardia spp., Granulibacter bethesdensis, Chromobacterium violaceum, and Francisella philomiragia are common bacterial pathogens. Aspergillus spp., Paecilomyces spp. as common fungus infections, and Candida or Trichosporon are common yeast pathogens in these patients. Spontaneous infection with other pathogens, such as mucormycosis, is rare. However, it is mainly seen in patients treated with strong immunosuppressive medication for weeks (3, 6, 7).
The common pulmonary radiological features of mucormycosis infections are multifocal or multilobar consolidation and numerous simple or cavitating nodules. Although "halo or reversed halo," "bird’s nest," or "black halo" signs are mentioned as classic signs of pulmonary mucormycosis infection in CT scans and magnetic resonance imaging (MRI), organ or chest wall invasion is evidence of aggressive infection. However, mass-like consolidation is an extremely rare presentation of mucormycosis infection. Similar to our study, Bhalla et al. confirmed a mucormycosis infection in the form of a mass-like consolidation in an 8-year-old CGD patient with respiratory symptoms who did not receive immunosuppression medication as well (8).
For follow-up, ESR remains a very sensitive laboratory test for ongoing infection. The major goal of the treatment of CGD patients is the prevention of life-threatening infections. Thus, they are usually treated with prophylaxis antibiotics, which, according to the literature, can reduce reinfection and hospitalization in patients (2, 4, 5, 10).
Until today, the only curative treatment for these patients is HSCT, with survival rates increasing to over 90% in patients. Bone marrow transplantation is a valuable technique leading to stable remission of CGD. Usually, low-intensity, no ablative transplantation of HLA-identical siblings in patients with CGD is preferred, though the setting of recurrent fungal infections in these patients reduces the risk of successful transplantation. However, some studies have suggested using leukocyte transfusions and gene therapy in severe infections, which are under trial. Gene therapy is an alternative treatment that provides an option for patients without an HLA-identical donor. Our patient was discharged with a prophylaxis antibiotic. Although she did not receive significant immunosuppressive medication, she was infected with mucormycosis. During the admission, she was treated with antifungal medication, and after three weeks, she was discharged and recommended doing HSCT (5-7, 9, 10).
Diagnosis of mucormycosis is often delayed and requires immediate medical and surgical intervention. In our case, disease pattern recognition and antifungal management were timely performed. Although mucormycosis infection usually causes complications, fortunately, our patient was discharged without any complications, which is a strength of our study. We faced some limitations during our case report study. Due to the limited facilities in our hospital, we had to send the sample of the lung biopsy to another center for analysis. Furthermore, due to the lack of specialized facilities for HSCT, we had to refer the patient to a specialized center for further treatment, which led to the fact that we could not have a proper treatment follow-up of the patient.
3.1. Conclusions
The chronic granulomatous disease is a primary immunodeficiency disease that can increase patients’ vulnerability to bacterial, fungal, or yeast infections. Although infection with mucormycosis is a rare condition, the possibility of different types of infectious pathogens is possible due to the immune system disorder in CGD patients. Physicians should pay attention to all the common and rare clinical and paraclinical evidence of these infections in patients to make a proper diagnosis and start suitable treatment to prevent further complications.