1. Background
Tetralogy of Fallot (TOF) is a common cyanotic congenital heart disease (1). The incidence of TOF is 7 - 10%, which happens in 0.5 per 1000 live births (1, 2). TOF consists of four element defects: ventricular septum defect, sub-pulmonary obstruction, right ventricular (RV) hypertrophy, and overriding aorta on the septum (2). The severity of RV outlet stenosis showed various degrees of cyanosis (3).
The American Society for congenital heart disease reported a 3% mortality in patients before repair surgery (4). Diagnostic instruments could identify all heart abnormalities before and after surgery (2). Before open heart surgery, the size, shape, and probable stenosis in the pulmonary artery, infundibulum obstruction, additional defects in the atrium of the ventricle septum, the proximal origin of the coronary artery, the origin, and size of the lungs' blood supply out of the heart, the shape and function of RV, and tricuspid must be specified (2). The physician should assess the volume and pressure of RV due to stenosis or regurgitation of tricuspid, systolic of RV and left ventricle (LV), postoperative scars, fibrosis, and RV aneurysm, aortic dilation or regurgitation after the surgery (2).
Cardiac magnetic resonance (CMR) without ionizing radiation provides details of RV function and structure compared to echocardiography (5). Electrocardiogram (ECG), as the non-invasive and accessible method at all levels of medical centers, can predict the risk of sudden death (6). Patients with repaired TOF (r TOF) are exposed to moderate to severe pulmonary regurgitation that gradually leads to progressive RV dilation, RV fibrosis, RV and LV dysfunction, and desynchronized ventricles (7). CMR presents all these findings with high accuracy, known as the gold standard but expensive method (8). Furthermore, less diastolic compliance can increase end-diastolic pressure and heart failure (9). Scars at the RV outlet result in conduction delay and arrhythmia in the ventricles (9). This complication presents tachypnea, tachycardia, murmur, syncope, and activity intolerance (5). Moreover, late RV complications were correlated to QRS duration, and the prolonged QRS (more than 180 ms) was related to increased mortality in patients (10).
Some studies mentioned a correlation between cardiovascular diseases and fragmented QRS (fQRS) (10). Fragmented QRS is defined as additional spikes within the QRS complex and indicates conduction disturbance due to myocardial scar or fibrosis (6). Although previous studies noted a significant correlation between fQRS and ventricular tachycardia and/or ventricular fibrillation (6, 10), the correlation between ECG parameters and CMR findings in RTFO is unknown. Notably, the pulmonary valve (PV) gradually needs to be replaced, and compared to other Valvulopathies, PV correlated with increased mortality and morbidity (11). Therefore, regarding the ECG and CMR guides, the specialist should decide the appropriate timing for pulmonary valve replacement (PVR).
2. Objectives
Due to the importance of these issues, we aimed to assess the correlation between clinical symptoms and ECG parameters with CMR findings.
3. Methods
This cross-sectional study assessed the r TOF in patients who underwent CMR between May 2020 and September 2022 at Imam Reza Hospital, Mashhad, Iran. Children aged less than 20 years were entered. Patients with other cardiac defect anomalies except patent ductus arteriosus (PDA) and/or atrial septal defect (ASD) were excluded. The pediatric cardiologist evaluated the patient's signs and symptoms, including tachycardia, tachypnea, murmur, fatigue, and chest pain. According to PVR criteria, patients were divided into three groups: patients who underwent surgery, patients who had the indication of surgery but had not undergone it yet, and patients with no indication of surgery (5).
The imaging was recorded on a 1.5-tesla Siemens Avanto B17 MRI system. The primary imaging with steady state free precession (SSFP) sequence and 8 - 16 consecutive sequences (5 mm thickness in each sequence) from base to apex were taken. The DuBois formula and Argus-Siemense software calculated all the ventricles' volume. The right ventricle outlet dimension, with the largest anterior-posterior size, which is perpendicular to the long axis at the end of the diastole, was measured. The values of ejection fraction (EF) and ventricle outlet according to EF = (EDV-ESV)/EDV and SV = (EDV-ESV) were obtained, respectively. Blood flow analysis was done in the imaging contrast phase to estimate the stenosis or insufficiency valve. The pulmonary valve regurgitation fraction was calculated from the reverse pulmonary flow volume over the forward pulmonary flow volume.
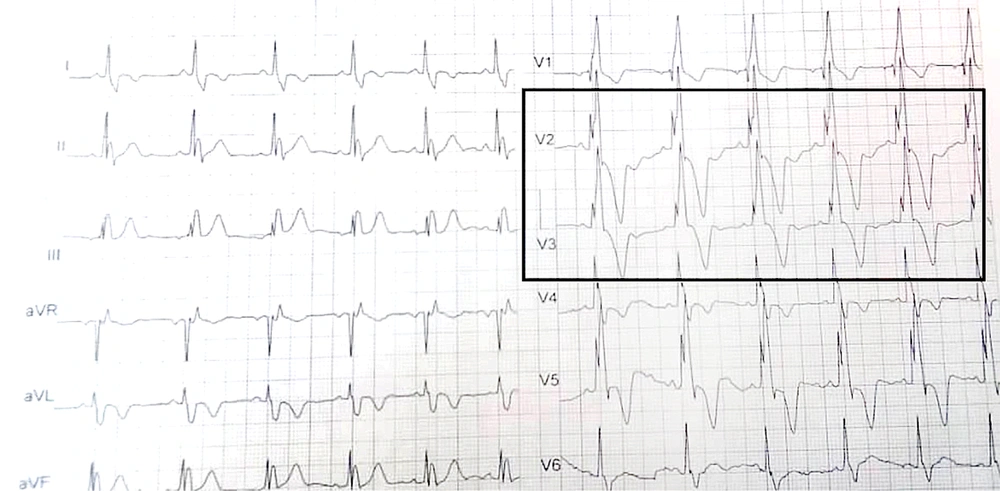
According to size and heartbeat, 20 - 30 sequences were recorded every 12 - 15 seconds. According to Tal Geva, RVESV > 80 mL/m2, RVEDV > 150 mL/m2, and RVEF < 47% as the right heart indexes are considered as the PVR indication and right cardiac dysfunction (12). Then, the 12-lead ECG was taken and magnified with Photoshop. A pediatric cardiologist interpreted the ECGs and recorded HR and pulse rate (PR) intervals. QT correction is calculated by Bazett's formula (QTc = QT / √RR). Fragmented QRS was defined as an additional R wave or notch in the nadir of the S wave in ≥ 2 contiguous leads (right-sided/septal: aVR, V1, V2; anterior: V2–V5; lateral: I, aVL, V5, V6; or inferior: II, aVF, III) in patients with QRS duration < 120 ms if the patients had bundle branch block, in two contiguous leads of R/S complex, three R waves/notch considered as fQRS (Figure 1).
3.1. Ethical Considerations
This study was approved by the Vice-Chancellor of Research at Mashhad University of Medical Sciences (Code: IR.MUMS.MEDICAL.REC.1400.441). The written informed consent letter was obtained from all parents/guardians.
3.2. Statistical Analysis
Data were reported by descriptive statistics. A pediatrician interpreted the ECGs. The odds Ratio (OR) and 95% confidence interval were calculated for analyses by using Statistical Package for Social Studies version 18. A P-value less than 0.05 is considered a significant correlation.
4. Results
Among a total of 42 rTOF patients, 20 were male. They had been assessed by CMR to determine PVR time. The mean age at CMR was 14.33 years old. Table 1 presents an overview of population characteristics with and without fQRS. In this study, 42.7% of patients had RV dysfunction, according to CMR, and in the ECG of 52.9% of patients who had indicated PVR, the fQRS was seen. We found a murmur in the clinical examination of 64.3% of patients. The most common sign was tachycardia and tachypnea.
| Variables | All (n = 42) | fQRS (n = 17) | NO fQRS (n = 25) |
|---|---|---|---|
| Mean age, (y) | 14.33 ± 5.1 | 13.76 ± 3.68 | 14 ± 5.9 |
| Gender (male) | 20 (47.6) | 12 (70.6) | 8 (32) |
| Age of repair surgery, (y) | 2.5 ± 2.27 | 1.96 ± 0.7 | 2.25 ± 1.57 |
| PVR | |||
| Yes | 11 (26.2) | 6 (35.3) | 5 (20) |
| No | 22 (52.4) | 2 (11.8) | 20 (80) |
| Indication | 9 (21.4) | 9 (52.9) | 0 |
| Murmur | |||
| Yes | 27 (64.3) | 13 (76.5) | 14 (56) |
| No | 15 (35.7) | 4 (23.5) | 11 (44) |
| Sign/symptoms | |||
| Tachycardia/tachypnea | 17 (40.5) | 6 (35.3) | 11 (44) |
| Chest pain | 7 (16.7) | 2 (11.8) | 5 (20) |
| Fatigue | 18 (42.9) | 9 (52.9) | 9 (36) |
Abbreviation: PVR, pulmonary valve replacement.
a Values are expressed as No. (%) or mean ± SD.
Low RVEF and high RVOT size had a significant correlation with the early detection of the murmur (Table 2). The Signs and symptoms in the clinical examination had a significant positive correlation with right heart parameters in CMR, such as RVEF, RVOT size, main PA flow, RPA flow, and RPA effective flow. Increased RVEDV was the only CMR parameter that strongly correlated with patients who indicated PVR. The HR increased at the same time as the increased LA and RA volume, RPA size, and flow. Although QTc had no significant correlation to CMR parameters, fQRS showed a positive correlation to RVEDV, RVESV, and RVEF.
| P-Value | ||||||
|---|---|---|---|---|---|---|
| Sign/Symptom | Murmur | HR | PRI | QTc | fQRS | |
| AAO volume | 0.001 | 0.525 | 0.097 | 0.566 | 0.898 | 0.833 |
| RVEDV | 0.815 | 0.723 | 0.728 | 0.904 | 0.549 | 0 |
| RVESV | 0.085 | 0.0.66 | 0.834 | 0.379 | 0.637 | 0 |
| RVEF | 0.038 | 0.019 | 0.597 | 0.690 | 0.640 | 0.005 |
| LVEF | 0.92 | 0.094 | 0.682 | 0.494 | 0.288 | 0.230 |
| RPA size | 0.548 | 0.4 | 0.024 | 0.134 | 0.382 | 0.913 |
| LPA size | 0.452 | 0.813 | 0.305 | 0.655 | 0.495 | 0.528 |
| Main PA size | 0.166 | 0.995 | 0.774 | 0.820 | 0.205 | 0.387 |
| RVOT size | 0.001 | 0.015 | 0.103 | 0.960 | 0.345 | 0.209 |
| LA volume | 0.173 | 0.916 | 0.021 | 0.980 | 0.771 | 0.601 |
| RA volume | 0.180 | 0.733 | 0.023 | 0.462 | 0.248 | 0.256 |
| Main PA flow | 0.007 | 0.431 | 0.091 | 0.934 | 0.131 | 0.764 |
| Main PA effective flow | 0.1 | 0.844 | 0.398 | 0.823 | 0.035 | 0.804 |
| RPA flow | 0.003 | 0.372 | 0.04 | 0.602 | 0.285 | 0.913 |
| RPA effective flow | 0.008 | 0.813 | 0.501 | 0.988 | 0.225 | 0.435 |
| LPA regurgitation fraction | 0.021 | 0.572 | 0.038 | 0.586 | 0.832 | 0.544 |
| RPA regurgitation fraction | 0.633 | 0.152 | 0.137 | 0.602 | 0.965 | 0.499 |
| LPA flow | 0.425 | 0.703 | 0.317 | 0.817 | 0.654 | 0.551 |
| LPA effective flow | 0.04 | 0.198 | 0.241 | 0.586 | 0.263 | 0.231 |
Abbreviations: AAO volume, ascending aortic volume; RVEDV, right ventricular end-diastolic volume; RVESV, right ventricular end-systolic volume; RVEF, Right ventricular ejection fraction; LVEF, left ventricular ejection fraction; RPA size, right pulmonary artery; LPA, left pulmonary artery; Main PA size, main pulmonary artery size; RVOT size, Right ventricular outflow tract; LA volume, left atrium volume; RA volume, right atrium volume; Main PA flow, main pulmonary artery flow; Main PA effective flow, main pulmonary artery effective flow; RPA flow, right pulmonary artery flow; LPA regurgitation fraction, Left pulmonary artery regurgitation fraction; RPA regurgitation, right pulmonary artery regurgitation fraction; LPA flow, left pulmonary artery flow; LPA effective flow, left pulmonary artery effective flow; HR, heart rate; PRI, PR interval; QTc, QT correction; fQRS, fragmented QRS.
Our analysis demonstrated fQRS was more seen in RVEDV more than 150 mL/m2 (OR 4.4, 95% CI 2.03 - 9.5; P-value = 0), RVESV more than 80 mL/m2 (OR 2.83, 95% CI 1.59 - 5.04; P-value = 0), and RVEF less than 47% (OR 2.06, 95% CI 1.45 - 2.93; P-value = 0.005). None of the patients had chest pain in PVR compared to patients who did not undergo surgery. The surgery at a younger age significantly correlated with lower AAO volume, RPA size, LPA size, RA and LA volumes, and main PA flow according to their age.
5. Discussion
In this study, we assessed the CMR of 42 rTOF patients. CMR is the most important imaging method for detecting PVR timing. It is an expensive but accurate method that can be used to obtain a quantitative evaluation in patients (13). Elsaka et al. recommended having a baseline CMR as their situation may change during 3 to 7 years (14). The repaired surgery at a younger age significantly decreased the atriums volume, pulmonary arteries size, and volume. We mentioned that the PVR improved the signs and symptoms of patients and prevented chest pain events. Re-surgery in rTOF changes the quality of life of patients. We assessed the HR, RR, chest pain, and fatigue in rTOF. Our study showed that fatigue was the most common complaint of our patients.
Most children die in infancy without repair surgery, but after introducing comprehensive surgical repair in 1955, the long-term outlook for these individuals has been improved (13). However, the hemodynamic burden of TOF could easily be tolerated during childhood, cardiac arrhythmias, exercise intolerance, heart failure, and mortality increase throughout the third decade following surgery (5).
Mortezaeian et al. showed the importance of repair surgery in improving oxygen saturation, low occurrence spell, and increased z-score of right and left pulmonary arteries (15).
Therefore, they need PVR surgery at an older age compared to others. After repair surgery, pulmonary regurgitation leads to ventricle dilation, dysfunction, and failure (16).
PVR is a new technique that can be used to resolve pulmonary stenosis or regurgitation (17). Based on the European Society of Cardiology, symptomatic patients with severe PR, pulmonary stenosis (PS), progressive RV dilation, and dysfunction have PVR indications (18). A previous study revealed that PVR decreased the right ventricle volume and improved clinical signs and symptoms (19). Symptomatic patients benefited from PVR because cardiac remodeling improved the symptoms (16). According to the previous studies, the RVESV and RV mass were the predictors compared to EDV for PVR (20, 21). Although CMR is the gold standard, evaluating symptoms is the primary and important step in the PVR decision.
It is undoubtedly clear that ECG is frequently performed during the annual examination of patients with TOF and is affordable, non-invasive, and easily accessible (22). According to recent research by Bokma et al.12, fQRS measured with a conventional 12-lead ECG can accurately predict death (10). Consistent with ours, another research found fQRS correlated with CMR parameters and related to RV dilation (7). This finding corroborates the ideas of Buntharikpornpun et al., who suggested that fQRS predicted changes in RV parameters in CMR (7). Also, the extent of fQRS in ECG leads correlated to increased RVEDV, RVESV, and low RVEF (7).
Also, we highlighted the correlation between fQRS with RVEF of less than 47%, RVEDV with more than 150 mL/m2, and RVESV with more than 80 mL/m2. According to PVR criteria, these three parameters decide the PVR, and in both genders, fQRS indicated cardiac disease (23). Vogels et al. showed an increased risk of ventricular arrhythmias in the presence of fQRS in ECG (24). Bokma et al. demonstrated the importance of fQRS compared to QT duration to predict mortality in adults with rTOF (10, 25).
Although we did not assess the prediction of mortality according to fQRS in our study, RV dysfunction had significantly associated with fQRS. Generally, fQRS correlated with the myocardial scar in various cardiac diseases such as coronary artery diseases (26), Brugada syndrome (27), arrhythmogenic right ventricular dysplasia cardiomyopathy (28), hypertrophic cardiomyopathy (6), Bechet’s disease (29). It has been proven that fQRS is associated with Heart failure progression (30).
5.1. Limitations
This study had some limitations. Our study included a small sample size. It would be better if the study was conducted over a longer period and with a larger sample size. Also, echocardiography findings should be used to determine the time of CMR.
5.2. Conclusions
Compared to other ECG parameters, fQRS could strongly predict RVESV, RVEDV, and RVEF as the right heart indexes. Besides, repair surgery at a younger age reduced the possibility of needing pulmonary valve replacement (PVR) surgery at older ages. Therefore, clinicians should consider these issues in patients.