1. Background
Kawasaki disease (KD) is an acute vasculitis that mainly affects children under the age of 5 years. Despite its increasing prevalence, the exact cause of KD is unknown. Diagnosis is based on fever and specific clinical signs. Coronary artery lesions (CALs) associated with KD are of special concern; however, timely treatment with intravenous immunoglobulin (IVIG) and aspirin has been shown to be effective in reducing their incidence. However, a subset of KD patients, referred to as IVIG non-responders, do not respond satisfactorily to initial or repeat IVIG therapy. These patients might require additional measures, such as corticosteroid therapy, infliximab therapy, plasma exchange, or cytotoxic drugs. The early detection of primary and recurrent IVIG resistance is crucial to reducing the incidence of CAL and, ultimately, the cost of therapy.
Despite advances in the understanding of KD, the exact etiology of the disease remains elusive, and a specific diagnostic test is not currently available. Procalcitonin (PCT), a marker associated with severe bacterial infection, is a promising potential marker for KD. Previous studies have reported variable levels of PCT in KD patients but were limited by small sample sizes and retrospective designs. This study aimed to investigate the relationship between PCT levels and severe outcomes, especially CALs, in KD children. There is little information on PCT as a marker of IVIG resistance in KD patients. Previous studies have shown variable levels of PCT. However, the aforementioned studies have utilized different doses of IVIG for treatment purposes, and they had retrospective designs. Although some studies have investigated serum PCT levels in KD patients, further studies are needed to understand the relationship between KD and PCT.
2. Objectives
Therefore, this study aimed to investigate PCT levels in children with KD and evaluate their association with severe outcomes, especially CAL.
3. Methods
3.1. Patients
All children diagnosed with KD and admitted to Namazi hospital in Shiraz, Iran, in 2019 were enrolled in this cross-sectional study. The parents of all patients provided a written informed consent form. The KD patients were categorized into complete and incomplete KD (cKD and iKD, respectively), according to the American Heart Association (AHA) criteria. A diagnosis of cKD was established if all diagnostic criteria were fulfilled. The diagnostic criteria were 1) the presence of fever for at least four days and 2) having four of the five principal clinical features of KD at minimum. On the other hand, iKD was diagnosed when there were fewer than three diagnostic criteria, together with fever. Additionally, patients' laboratory results should be typical of KD, and there was no differential diagnosis.
Patients were included in the study if a blood sample was collected before the 10th day of starting the disease and before IVIG administration and the collection of biological samples. However, patients with clinically recognizable myocarditis or a known congenital heart disease were excluded. The IVIG resistance was defined as being febrile for 36 hours after the initial IVIG administration or having recurrent fever. Fever was defined as a body temperature above 38°C.
3.2. Two-Dimensional Echocardiography
Two-dimensional echocardiography was performed for all KD patients on the day of diagnosis before treatment. All the scans were examined regarding pericardial effusion, CAL, and mitral regurgitation. The left ventricular function, including the ejection fraction, was also assessed. The electrocardiographic rhythm and the heart rate were measured during the examinations. A coronary artery was regarded abnormal if the internal lumen diameter was > 2.5 mm in the age group of < 3 years, > 3 mm in the age group of 3 - 9 years, and > 3.5 mm in the age group of 9 - 14 years. Additionally, the internal diameter of the abnormal coronary arterial segment was ≥ 1.5 times larger than that of the adjacent normal segment, and there was an irregular lumen. The size of all coronary arteries was converted to a z-score for comparison of dilatation in individuals. The z-scores were assessed online (http://www.parameterz.com/sites/coronary-arteries). Coronary artery dilatation was categorized as small (z-score of 2.5 - 5), large (z-score of 5 - 10), and giant (z-score > 10) (or the presence of ectasia or aneurysms). The patients were divided into two groups depending on the presence of coronary artery abnormalities (CAAs) (1).
3.3. Peripheral Venous Blood Examinations
Peripheral venous blood was collected to measure the baseline serum PCT levels in cKD and iKD patients on day 0. Moreover, blood culture, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), complete blood cell (CBC) count, aspartate aminotransferase (AST), alanine transaminase (ALT), total protein, albumin, sodium, blood urea nitrogen (BUN), and creatinine were measured. A urinalysis was also performed. Then, the D0 values (one day before the onset of treatment) of cKD and iKD patients were compared.
3.4. Treatment
All patients received IVIG at 2 g/kg for 10 - 12 hours and oral aspirin at 80 - 100 mg/kg/day. The oral aspirin was continued every 6 hours until the patient was afebrile for at least 48 hours; then, it decreased to 3 - 5 mg/kg/day for 8 weeks. This study was approved by the Research Ethics Committee of the Shiraz University of Medical Sciences (reference number: IR.SUMS.MED.REC.1398.637).
3.5. Statistical Analysis
The data were analyzed using Stata software version 14.0. We used mean (standard deviation [SD]) and number (%) to describe quantitative and qualitative variables, respectively. Then, the univariate and multivariate logistic regression model was used to determine the predictive factors of complete KD, resistance to IVIG, and occurrence of coronary complications. Moreover, crude and adjusted odds ratio (OR) with a 95% confidence interval (CI) were estimated. To determine the best cut-off point of PCT and the occurrence of coronary complications in patients with KD, the receiver operating characteristic (ROC) curve was drawn. Then, the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and the area under the ROC curve (AUC) were estimated. A P-value < 0.05 was considered as a significant level.
4. Results
This cross-sectional study aimed to assess serum PCT levels in patients with complete and incomplete KD at Namazi hospital in Shiraz in 2019. A total of 68 children with suspected KD and a two-week follow-up were considered eligible for this study. However, 30 children were excluded due to the spontaneous resolution of pyrexia without IVIG or the lack of PCT measurements before IVIG infusion.
Finally, 38 patients were included in this study, with 23 and 15 patients having incomplete and complete KD, respectively. Table 1 shows the mean age and PCT levels in patients with complete and incomplete KD were 3.44 ± 3.20 vs. 2.67 ± 2.13 years and 3.07 ± 2.87 vs. 2.40 ± 2.31 ng/mL, respectively. Additionally, in the complete and incomplete groups, 80% and 69.6% of the patients were male, respectively. The levels of other laboratory parameters in terms of complete and incomplete Kawasaki can be seen in Table 1.
| Quantitative Variables and Type of Kawasaki | Number | Mean ± SD |
|---|---|---|
| Age (y) | ||
| Incomplete | 23 | 3.44 ± 3.20 |
| Complete | 15 | 2.67 ± 2.13 |
| PCT | ||
| Incomplete | 23 | 3.07 ± 2.87 |
| Complete | 15 | 2.40 ± 2.31 |
| ESR | ||
| Incomplete | 23 | 71.3 ± 33.75 |
| Complete | 15 | 58.9 ± 28.99 |
| CRP | ||
| Incomplete | 23 | 95.13 ± 55.15 |
| Complete | 15 | 78.66 ± 60.08 |
| WBC | ||
| Incomplete | 23 | 12743.48 ± 5859.63 |
| Complete | 15 | 14833.33 ± 5949.38 |
| N/L | ||
| Incomplete | 23 | 2.10 ± 1.47 |
| Complete | 15 | 3.86 ± 3.84 |
| Hb | ||
| Incomplete | 23 | 10.1 ± 0.93 |
| Complete | 15 | 10.29 ± 0.80 |
| Plt | ||
| Incomplete | 23 | 428391.30 ± 228232.08 |
| Complete | 15 | 411800.00 ± 295708.64 |
| ALT | ||
| Incomplete | 23 | 30.04 ± 30.57 |
| Complete | 15 | 31.33 ± 30.17 |
| Alb | ||
| Incomplete | 23 | 3.55 ± 0.42 |
| Complete | 15 | 3.58 ± 0.55 |
| Qualitative Variables | Type of Kawasaki | |
| Incomplete (%) | Complete (%) | |
| Gender | ||
| Female | 7 (30.4) | 3 (20) |
| Male | 16 (69.6) | 12 (80) |
Demographic Characteristics and Laboratory Parameters of Patients According to Type of Kawasaki
Table 2 shows that the mean age and PCT levels in resistant and responsive patients to IVIG were 2.61 ± 2.20 vs. 3.22 ± 2.92 years and 4.64 ± 2.70 vs. 2.35 ± 2.72 ng/mL, respectively. Furthermore, 100% and 69.7% of the patients were male in the resistant and responsive patients to IVIG, respectively.
| Quantitative Variables and Resistant to IVIG | Number | Mean ± SD |
|---|---|---|
| Age (y) | ||
| Yes | 5 | 2.61 ± 2.20 |
| No | 33 | 3.22 ± 2.92 |
| PCT | ||
| Yes | 5 | 4.64 ± 2.70 |
| No | 33 | 2.35 ± 2.72 |
| ESR | ||
| Yes | 5 | 78.40 ± 54.93 |
| No | 33 | 64.57 ± 28.14 |
| CRP | ||
| Yes | 5 | 80.80 ± 64.06 |
| No | 33 | 89.90 ± 56.77 |
| WBC | ||
| Yes | 5 | 13800.00 ± 5436.45 |
| No | 33 | 13533.33 ± 6118.04 |
| N/L | ||
| Yes | 5 | 4.61 ± 6.10 |
| No | 33 | 2.53 ± 1.89 |
| Hb | ||
| Yes | 5 | 9.66 ± 0.72 |
| No | 33 | 10.25 ± 0.88 |
| Plt | ||
| Yes | 5 | 430200.00 ± 179459.46 |
| No | 33 | 420575.76 ± 264779.51 |
| ALT | ||
| Yes | 5 | 26.20 ± 11.98 |
| No | 33 | 31.21 ± 31.94 |
| Alb | ||
| Yes | 5 | 3.20 ± 0.33 |
| No | 33 | 3.62 ± 0.47 |
| Qualitative Variables | Resistant to IVIG | |
| Yes (%) | No (%) | |
| Gender | ||
| Female | 0 (0) | 10 (30.3) |
| Male | 5 (100) | 23 (69.7) |
Demographic Characteristics and Laboratory Parameters of Patients According to Resistant and Responsive to Intravenous Immunoglobulin (IVIG)
Table 3 shows the mean PCT levels were 4.37 ± 3.15 and 1.39 ± 2.58 ng/mL in patients with coronary and without complications, respectively. The mean age was 2.94 ± 2.52 and 3.28 ± 3.07 years, respectively. Moreover, 93.7% and 59.1% of the patients were male in the patients with coronary and without complications, respectively.
| Quantitative Variables and Coronary Complications | Number | Mean ± SD |
|---|---|---|
| Age (y) | ||
| No | 22 | 3.28 ± 3.07 |
| Yes | 16 | 2.94 ± 2.52 |
| PCT | ||
| No | 22 | 1.39 ± 2.58 |
| Yes | 16 | 4.37 ± 3.15 |
| ESR | ||
| No | 22 | 64.45 ± 30.94 |
| Yes | 16 | 69.06 ± 34.58 |
| CRP | ||
| No | 22 | 85.00 ± 53.97 |
| Yes | 16 | 93.62 ± 62.21 |
| WBC | ||
| No | 22 | 11831.82 ± 5135.71 |
| Yes | 16 | 15956.25 ± 6350.00 |
| N/L | ||
| No | 22 | 2.56 ± 1.82 |
| Yes | 16 | 3.41 ± 3.74 |
| Hb | ||
| No | 22 | 10.24 ± 0.96 |
| Yes | 16 | 10.07 ± 0.76 |
| Plt | ||
| No | 22 | 396990.90 ± 222201.09 |
| Yes | 16 | 457250.00 ± 294527.75 |
| ALT | ||
| No | 22 | 31.45 ± 28.96 |
| Yes | 16 | 29.31 ± 32.31 |
| Alb | ||
| No | 22 | 3.68 ± 0.38 |
| Yes | 16 | 3.39 ± 0.54 |
| Qualitative Variables | Coronary Complications | |
| No (%) | Yes (%) | |
| Gender | ||
| Female | 9 (40.9) | 1 (6.3) |
| Male | 13 (59.1) | 15 (93.7) |
Demographic Characteristics and Laboratory Parameters of Patients According to Coronary Complications
The results of the multivariable logistic regression model after removing the effect of possible confounding variables showed that PCT is not a significant predictive factor in having complete KD (OR = 0.78; 95% CI: 0.56-1.08; P = 0.143) nor for resistance to IVIG (P > 0.05).
Finally, Table 4 shows that the results of the multivariate regression model reveal PCT as a significant predictive factor in the occurrence of coronary complications in patients with KD; accordingly, for one unit increase in PCT level, the chance of coronary complications increases 2.03 times (OR = 2.03; 95% CI: 1.12 - 3.67; P = 0.019).
| Variables | Univariate Logistic Regression | Multivariate Logistic Regression | ||
|---|---|---|---|---|
| OR crude (95% Cl) | P-Value | OR adjusted (95% Cl) | P-Value | |
| Gender | ||||
| Female | Reference | 0.037 | Reference | 0.076 |
| Male | 10.34 (1.15 - 19.29) | 5.75 (0.29 - 11.44) | ||
| Age | 0.95 (0.75 - 1.21) | 0.706 | - | - |
| PCT | 2.17 (1.31 - 3.57) | 0.009 | 2.03 (1.12 -3.67) | 0.019 |
| ESR | 1.00 (0.98 - 1.02) | 0.659 | - | - |
| CRP | 1.00 (0.99 - 1.01) | 0.641 | - | - |
| WBC | 1.02 (1.01 - 1.06) | 0.045 | 1.00 (0.99 - 1.01) | 0.502 |
| N/L | 1.08 (0.85 - 1.37) | 0.528 | - | - |
| Hb | 0.80 (0.38 - 1.70) | 0.561 | - | - |
| Plt | 1.00 (0.99 - 1.04) | 0.462 | - | - |
| ALT | 0.99 (0.97 - 1.02) | 0.826 | - | - |
| Alb | 0.23 (0.05 - 1.11) | 0.067 | 0.29 (0.05 - 1.65) | 0.163 |
Predictive Factors of Coronary Complications by Univariate and Multivariate Logistic Regression a
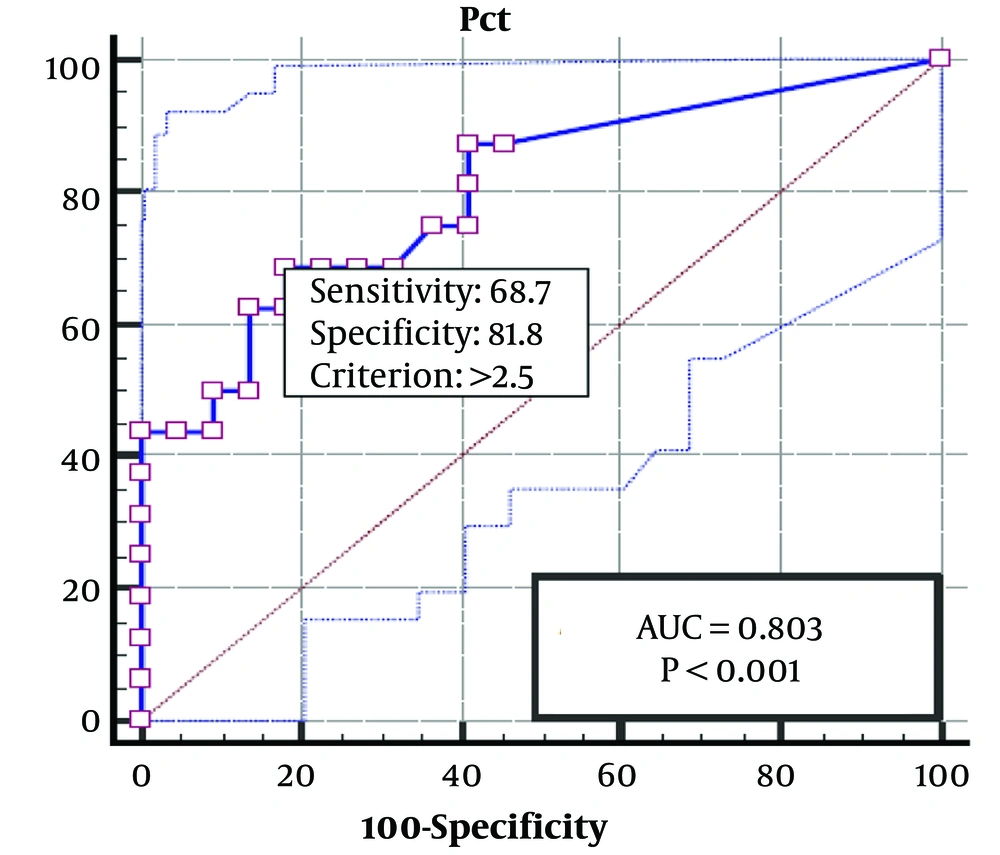
Then, to determine the best cut-off point of PCT and the occurrence of coronary complications in patients with KD, an ROC curve was drawn, and the best PCT cut-off was determined to be around 2.50 ng/Ml. As can be seen, the sensitivity, specificity, PPV, and NPV of this cut-off were determined to be 81.8, 68.7, 73.5, and 78.3%, respectively. In addition, the AUC was 0.803 (95% Cl: 0.756 - 0.866), which indicates the high accuracy of PCT in the occurrence of coronary complications in Kawasaki patients (Figure 1).
5. Discussion
This study examined the relation between serum PCT levels in children with KD and the incomplete disease pattern, unresponsiveness to IVIG, and coronary complications. Based on the present findings, the PCT level was a more suitable marker for KD severity than CRP and white blood cell count (WBC). Procalcitonin production can be induced in many non-hepatic tissues during inflammatory processes; however, CRP formation occurs in hepatic cells. Considering this mechanism of production, besides systemic vasculitis in KD, PCT is more closely associated with KD severity than CRP (2).
White blood cell count is often increased during inflammation. While WBC is normal, there are increasing or decreasing during infectious processes. Accordingly, the use of total WBC in KD is less common than in PCT or CRP. The level of CRP is associated with disease severity and CAL development. C-reactive protein can be used as a biomarker for identifying non-responders. Nonetheless, in the present study, neither CRP concentrations nor the development of CAL were significantly different between the IVIG non-responders and responders (3).
Although the exact etiology of KD remains unknown, it might arise from a genetic predisposition, along with an infection through an autoimmune mechanism or an undefined trigger. The cytokine profile, which represents the disease severity, is linked to CAL, indicating the role of serum PCT concentrations in CAL prediction (2).
In KD patients, the predictive role of serum PCT concentration in primary CAL is currently limited. The relationship between the serum PCT level and the development of CAL was assessed in the present study. A cut-off value of 2.5 ng/mL for CAL showed 81.8% sensitivity, 68.7% specificity, 73.5% PPV, and 78.3% NPV. Compared to the non-CAL group, the serum PCT level significantly increased in the CAL group.
In this regard, Okada et al. examined 25 KD patients, including 4 patients with CAA. The PCT concentration could represent KD severity, and a PCT concentration > 3.0 ng/mL was associated with CAA. On the other hand, Catalano-Pons et al. studied 18 KD patients, including 6 patients with CAA, and reported that a high PCT level was not related to the development of CAAs. It is worth mentioning that different definitions were used for CAA in these two studies. Additionally, different treatments were employed in these studies. Okada et al. treated patients with 400 mg/kg of IVIG for 5 days; however, Catalano-Pons et al. used 1 g/kg of IVIG for 2 days (4, 5).
Moreover, Dominguez et al. observed that a serum PCT level ≥ 0.5 or 1.0 ng/mL was associated with unresponsiveness to IVIG, which was lower in their study than in the present research, although both studies used the same measurement method; this discrepancy can be explained by differences in the measurement time. Additionally, Dominguez et al. assessed PCT by day 10 of the disease; nevertheless, in the current study, PCT was assessed at diagnosis (mostly within 5 days of the disease) (1).
Niu observed that the PCT concentrations below 0.25 ng/mL might be helpful for discriminating KD from sepsis, and the PCT concentrations of 0.25 - 0.50 ng/mL might be useful in predicting IVIG non-responsiveness. They also did not find any relation between PCT level and CAA development (6).
In the present study, the rate of aneurysm, despite appropriate treatment, was relatively high. Compared to other countries, Iranian studies have reported higher rates of coronary artery involvement, which could be due to the multi-factorial nature of KD and the genetic background of the Iranian population (7). The current study has some limitations. Probably the most important limitation of this study is its cross-sectional nature because these studies measure exposure and outcome simultaneously, and a detailed investigation of cause and effect relationships is not possible due to the lack of a temporal relationship between exposure and outcome. The second limitation of the current study is its single-center nature and small sample size, necessitating multi-center prospective cohort studies with a high sample size in future studies.
5.1. Conclusions
In conclusion, supplementary research is needed to determine the sensitivity and specificity of PCT for the diagnosis and prognosis of KD. Procalcitonin might be of value in predicting which children are at increased risk for CALs to intensify therapy.