1. Background
Overweight and obesity are defined as abnormal or excessive accumulation of fat that may impair health. Obesity in children is a complex health issue caused by many etiologies, such as genetic factors, inactivity, inappropriate eating patterns (or a combination of these factors), and in rare cases, it occurs due to hormonal problems. Although obesity and overweight have a hereditary basis, not all children in families with a history of obesity will be overweight. It is noteworthy that the risk of obesity is higher in children who have obese parents or siblings (1). The global burden of disease has predicted that approximately 124 million and 268 million children will be respectively obese or overweight by the year 2025 (2). Childhood obesity can harm the body in many ways. It can induce high blood pressure and high cholesterol, impaired glucose tolerance, and lead to type 2 diabetes, respiratory problems such as asthma and sleep apnea, musculoskeletal system problems, fatty liver disease, gallstones, and reflux in obese children (3-9). Furthermore, obesity is one of the causes of insulin resistance (IR) and is a major risk factor for diabetes. Abnormal fat deposition in skeletal muscle is known as the mechanism of IR associated with obesity (10).
IR develops when normal or high insulin levels produce a poor biological response (11). It is a decrease in the ability of insulin to act effectively on target tissues, especially muscles, liver, and fat (12). It is one of the underlying causes of diabetes and one of the symptoms of this disease, and it can be due to glucose metabolism disorders in skeletal muscles or the lack of inhibition of hepatic glucose production (13). Early detection of IR as an independent risk factor in cardiovascular complications and its changes is important in terms of prognosis (14). Compensatory hyperinsulinemia is when the body stimulates beta cells and secretes more insulin to regulate blood glucose levels in response to peripheral IR created in muscle and fat tissues (15). This mechanism may ultimately lead to pancreatic insufficiency. Prospective studies have shown that type 2 diabetes occurs when pancreatic beta cells cannot compensate for IR. The pathophysiology of IR, in addition, to type 2 diabetes, leads to obesity, atherosclerosis, hypertension, and dyslipidemia. Atherogenic dyslipidemia (hypertriglyceridemia, increased secretion of very low-density lipoprotein (VLDL) from the liver, and hypertriglyceridemia) with conditions of IR, increases secretion of very low-density lipoprotein (VLDL) from the liver, atherogenic low-density lipoprotein (LDL) and decrease high-density lipoprotein cholesterol (HDL) and has anti-atheroma properties (16-19).
Nettle (Urtica dioica L.) is known as a traditional medicine in the world which is considered a nutrient. It is named nettle in English and belongs to the Urticaceae family. This plant has different species, including big nettle, small nettle, and Mina flower (4, 20). It is abundant in North America, Northern Europe, and most of Asia (3). Urtica dioica has been used for allergies, prostatic hyperplasia, and many different disorders such as bladder infection, urinary tract inflammation, prostatic hypertrophy, seasonal allergies, especially in children, dandruff, acne, and treatment of rheumatic pain as well as diabetes mellitus (4, 20). Most of the studies conducted on the effects of nettle in reducing IR are in type 2 diabetes patients (21-24), and in general, studies on obesity in adults or animals have shown the effect of nettle on insulin sensitivity, hypoglycemia, and treating diabetes (25). Furthermore, animal studies have demonstrated that nettle can reduce IR and increase insulin growth factor-1.
2. Objectives
Based on the importance of using safe drugs and nutrients in childhood, the high prevalence of diabetes in children, the shortage of evidence on children with obesity, the limited human studies, and the contradictory results of previous research, this study aimed to investigate the effect of nettle extract on IR and metabolic parameters in obese children over 12 years of age.
3. Methods
3.1. Participants and settings
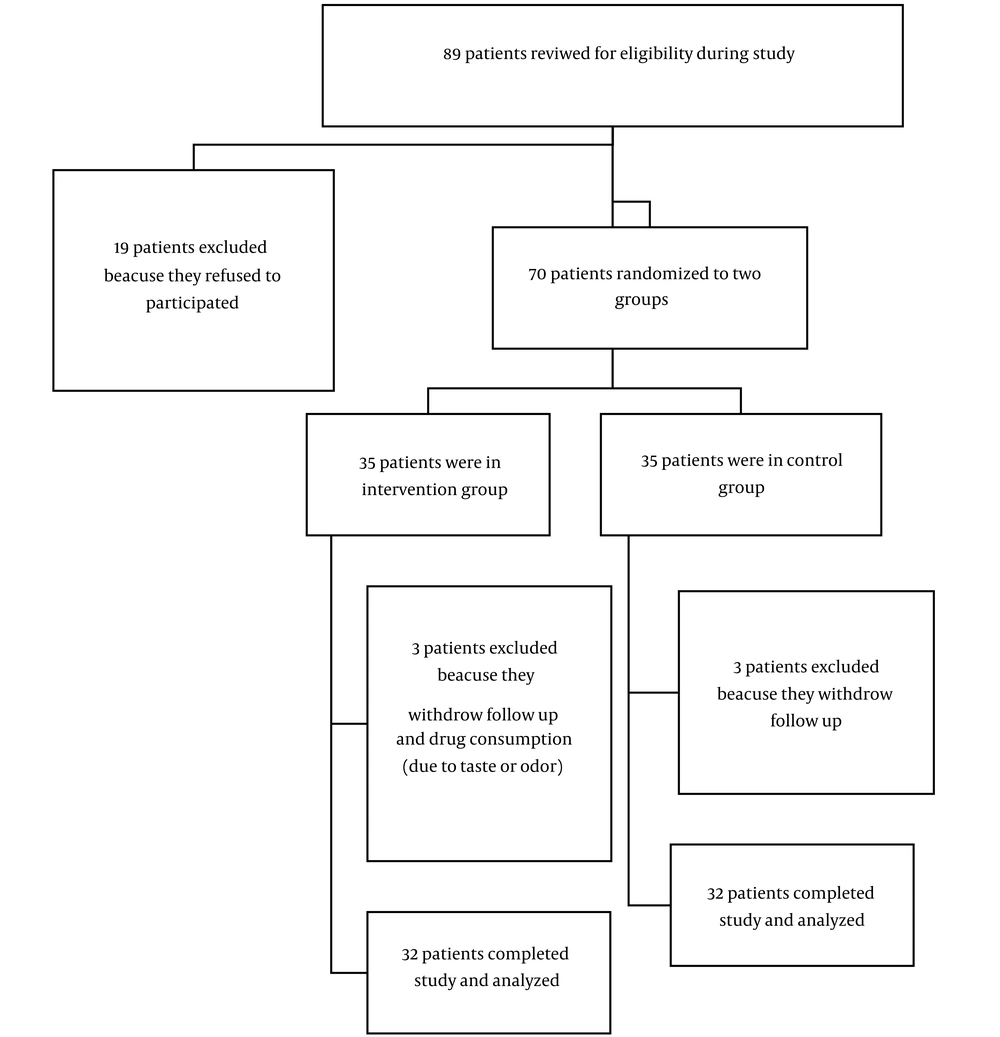
This study was a parallel randomized, single-blind, controlled clinical trial on patients referred to the endocrinology clinic of 17 Shahrivar Hospital in Rasht, Iran. The inclusion criteria were age 12 - 18 years and the presence of overweight and obesity (BMI 85 - 95% for age and sex was considered overweight, ≥ 95% for age and sex was considered obesity) (Figure 1).
3.2. Data Gathering
Data were gathered by a checklist, including demographic characteristics (e.g., age, sex, and concomitant diseases), anthropometric indices (weight and waist circumference (WC)), and laboratory parameters. Fasting blood sugar, LDL, HDL, TG, total cholesterol, and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) were measured at the beginning of the study, after 8 weeks, and after 12 weeks in both groups of patients. Anthropometric indices were indicated at the baseline and 12 weeks.
In HOMA, fasting blood sugar and fasting insulin were used to measure IR according to the following equation: HOMA-IR = ((fasting insulin (µU/mL)) × (fasting glucose (mmol/l))) / 22.5. In a healthy person, fasting plasma insulin was 5 µU/mL, and fasting plasma glucose was 4.5 mmol/l, the product of which was 22.5 times. On the other hand, the value of 22.5 was a normalizing factor for a healthy person. Therefore, HOMA equals 1 for a person with normal insulin sensitivity (21).
At the end of the study period, the patients were checked by telephone regarding drug use and side effects, and also a form with days of the week for 12 weeks was given to patients to record possible side effects. If patients had complications, they could call the number provided to them, and in case of serious side effects (such as stomach problems) or intolerance, they were excluded from the study. Also, patients with gastrointestinal problems (for example, reflux, etc.) and those who were non-compliant with the diet and medication were excluded.
3.3. Blinding and Randomization
In this study, only the statistician was blind. Due to the special taste and smell of nettle, despite the preparation of numerous formulations, we could not prepare a placebo.
Patients were randomly divided by block randomization method of 4 into two groups 32 after obtaining informed consent from the patients and parents or guardians.
3.4. Intervention and Control Groups
The intervention group received nettle syrup plus a specific diet, and the control group received the diet alone. Nettle syrup was prepared by Zardband pharmaceutical company, Iran and prescribed to patients in the form of 5 cc twice a day (according to the manufacturer's information, it contained the active ingredient quercetin based on at least 0.04 mg/mL of chlorogenic acid). Considering the difference in eating habits of the children and the families, the frequency of consumption, the child's inclinations, and combining meals, a diet that consisted of 55% carbohydrates, 30% fat (10% saturated and 20% unsaturated), and, 15% protein was administered with the help of a nutritionist.
Considering the potential effect of physical activity on obesity, the endocrinologist recommended moderate physical activity three times a week, for at least half an hour each time for both groups.
3.5. Sample Size
Based on the previous study (21) with a statistical power of 80%, a level of error of 0.05, and a standard deviation of 39.57 (intervention group) and 38.60 (control group), and d of 28.91, the minimum sample size including 10% drop rate was calculated as 32 for each group.
3.6. Ethical Considerations
Written informed consent letter was obtained from patients, parents, or guardians. This study was approved by the ethics committee of Guilan University of Medical Sciences (number: IR.GUMS.REC.1401.458 date: 2022-12-07) and was registered in the Iranian registry of clinical trials (IRCT20220516054879N3).
3.7. Statistical Analysis
This research used descriptive statistics such as mean, standard deviation, number, and percent to describe the obtained data. Also, the presuppositions related to the parametric tests were first examined to assess the research hypotheses. For this purpose, the normality of the research data was investigated using the Shapiro-Wilk test. After that, the homogeneity of the variance of the studied groups was checked using Levon's test. To analyze the research data for quantitative variables, independent samples, paired samples t-tests, and repeated measures with Greenhouse-Geisser and Huynh-Feldt correction were used. Also, Bonferroni's pairwise comparison test was applied. Qualitative variables were compared by the Pearson chi-square. It should be added that these calculations were done by IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp, and the significance level was considered 0.05.
4. Results
In this study, 35 patients in each group were enrolled. After excluding 3 patients in the control group due to withdrawing follow-up and 3 patients from the intervention group who refused to consume the drug because of the taste, odor, or gastric pain, the results were recorded based on 32 patients in each group. Most patients in the intervention and control groups were girls, with a frequency of 18 (56.3%) and 17 (53.1%), respectively. The mean age in the intervention and control groups were 13.84 ± 2.22 and 13.78 ± 2.38 months, respectively. There was no statistically significant relationship between groups regarding age (P = 0.457) and sex (0.802).
Based on the results, 19 (63.3%) patients in the control group and 15 (48.4%) in the intervention group had no underlying diseases such as pseudohypoparathyroidism and syndromes leading to obesity such as Turner syndrome, Prader-Willi, Cushing, etc. and no significant difference was noted. The highest frequency of drug consumption was dietary supplements, including omega 3, vitamin D, vitamin E, and ferrous sulfate, with a frequency of 22 people (68.8%) in the control group and 18 (56.3%) in the intervention group and no significant difference was noted between the two groups (P = 0.564).
In the intra-group analyses, the result showed no significant difference regarding FBS, weight, TG, LDL, HDL, TChol, Fasting insulin level, and HOMA-IR in the control group (P > 0.05). Despite no significant differences in terms of FBS, weight, TG, HDL, or fasting insulin levels in the intervention group (P > 0.05), a significant difference was observed in terms of LDL, TChol, and HOMA-IR (P = 0.003, P < 0.001, & P < 0.001, respectively).
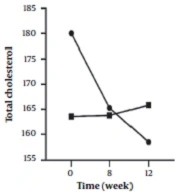
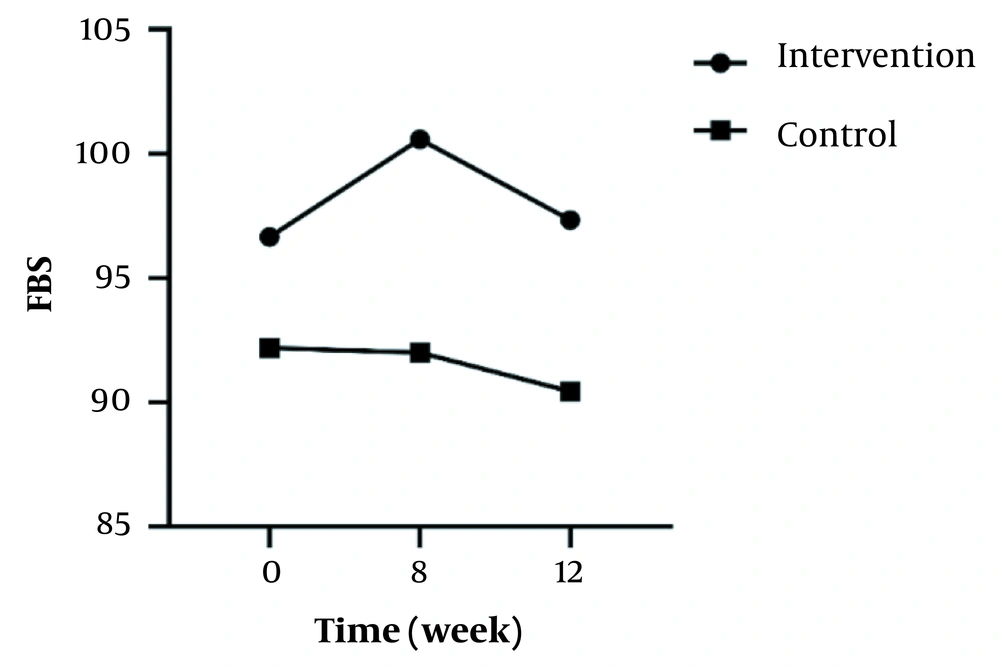
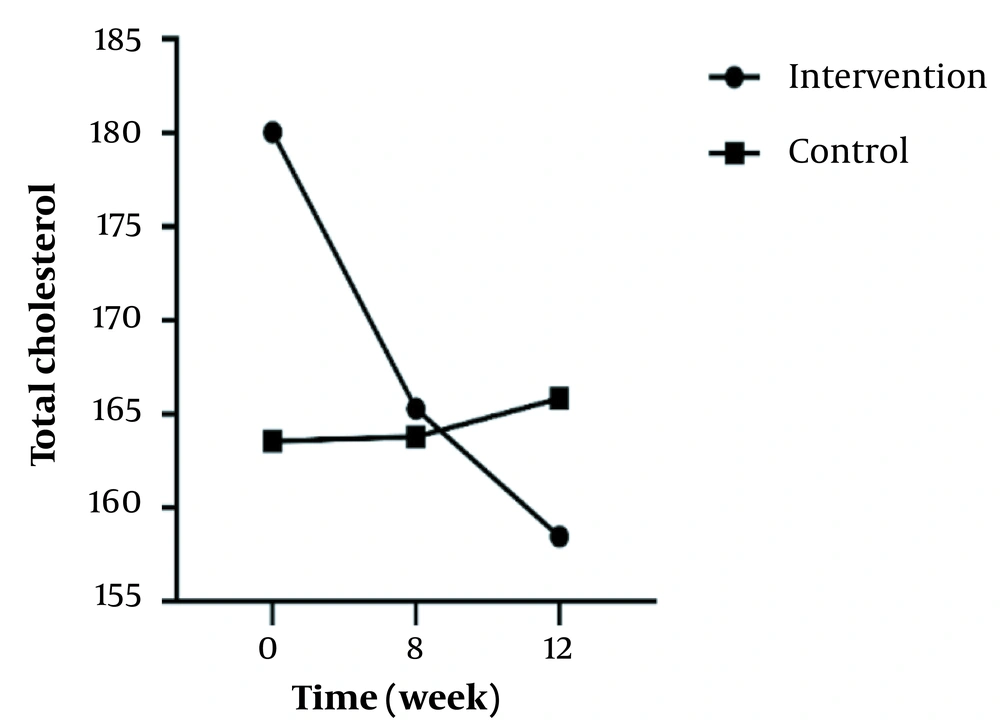
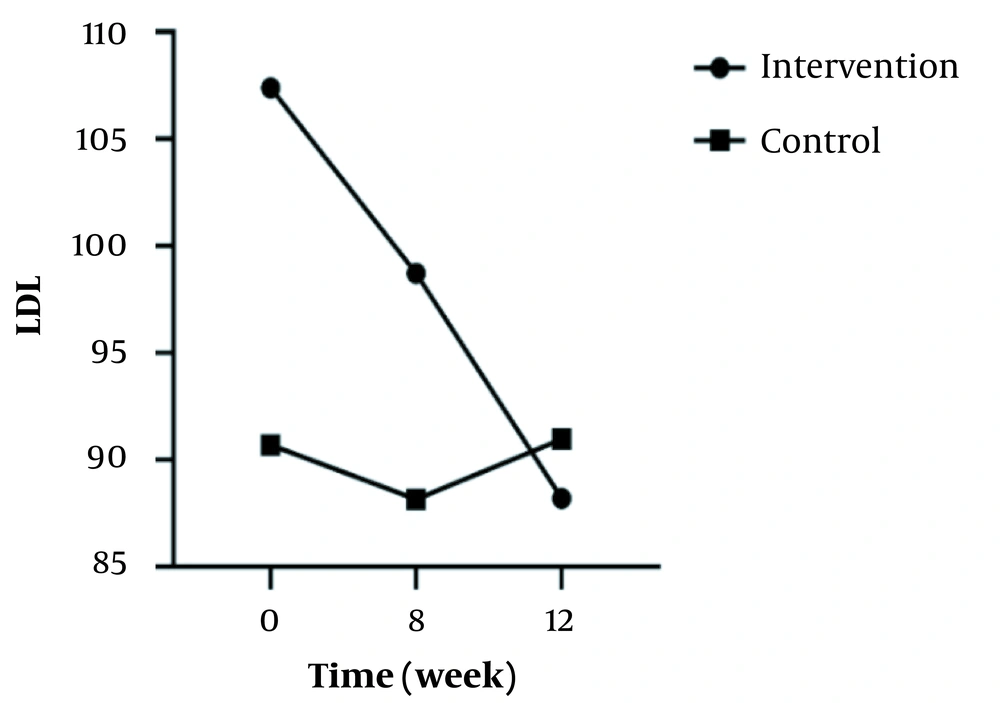
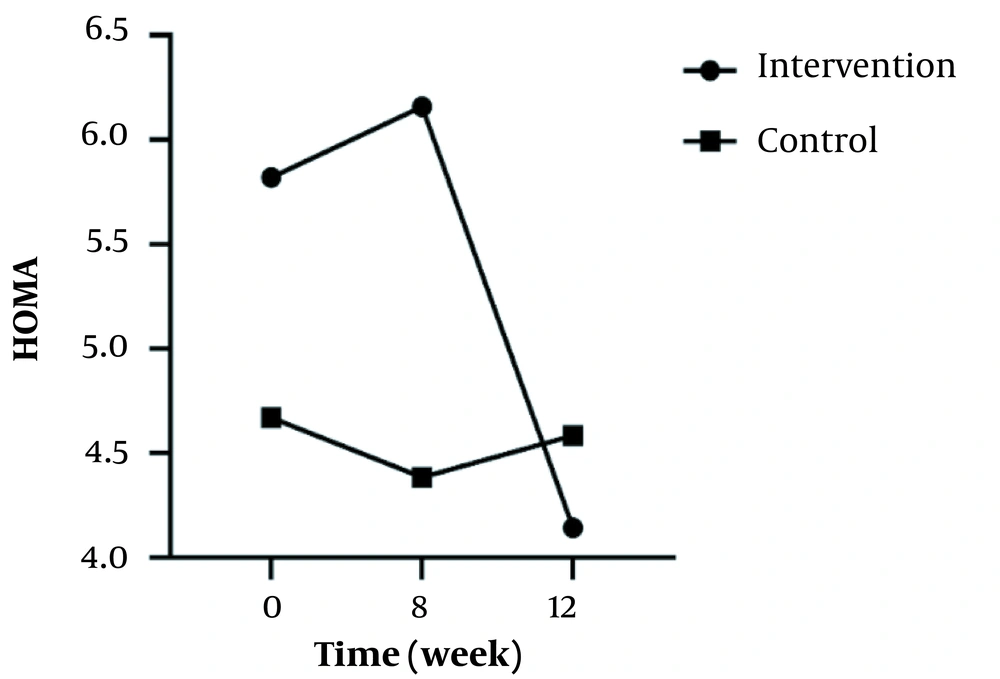
In the inter-group analyses, results showed significant differences in terms of FBS (P = 0.043), LDL (P = 0.023), and HOMA-IR (P = 0.025) at the eight weeks of the study. They indicated that these variables were significantly higher in the intervention group compared to the control group. Besides, only FBS (P = 0.044) had a significant difference at twelve weeks and was higher in the intervention group 8 (Table 1). Figures 2 - 5 shows the FBS, TChol, and HOMA-IR trends in the intervention and control groups, respectively.
| Variables | Baseline | 8 Weeks | 12 Weeks | P-Value a |
|---|---|---|---|---|
| FBS | ||||
| Intervention | 96.66 ± 18.72 | 100.59 ± 26.10 | 97.34 ± 20.02 | 0.331 |
| Control | 92.19 ± 8.03 | 92 ± 9.21 | 90.44 ± 10.48 | 0.466 |
| Inter-group P-value b | - | 0.043 | 0.044 | - |
| Weight | ||||
| Intervention | 67.76 ± 14.11 | - | 67.32 ± 15.54 | 0.598 |
| Control | 71.41 ± 17.83 | - | 72.30 ± 17.53 | 0.330 |
| Inter-group P-value b | - | - | 0.117 | |
| TG | ||||
| Intervention | 158.16 ± 81.41 | 163.09 ± 75.90 | 154.53 ± 54.82 | 0.729 |
| Control | 166.63 ± 65.41 | 162.84 ± 70.67 | 160.53 ± 63.97 | 0.817 |
| Inter-group P-value b | - | 0.495 | 0.344 | - |
| LDL | ||||
| Intervention | 107.38 ± 37.82 | 98.72 ± 19.61 | 88.19 ± 19.51 | 0.003 |
| Control | 90.69 ± 23.24 | 88.16 ± 21.79 | 90.97 ± 19.62 | 0.759 |
| Inter-group P-value b | - | 0.023 | 0.286 | - |
| HDL | ||||
| Intervention | 45.72 ± 14.36 | 41.59 ± 11.27 | 44 ± 7.40 | 0.247 |
| Control | 43.52 ± 8.33 | 40.63 ± 7.48 | 42.31 ± 9.62 | 0.201 |
| Inter-group P-value b | - | 0.343 | 0.217 | - |
| T Chol | ||||
| Intervention | 180.03 ± 32.84 | 165.28 ± 21.57 | 158.47 ± 22.37 | < 0.001 |
| Control | 163.56 ± 27.74 | 163.78 ± 32.26 | 165.84 ± 27.31 | 0.889 |
| Inter-group P-value b | - | 0.828 | 0.242 | - |
| Fasting insulin levels | ||||
| Intervention | 22.99 ± 12.78 | 20.02 ± 11.32 | 18.15 ± 13.10 | 0.081 |
| Control | 20.52 ± 11.88 | 19.41 ± 10.92 | 20.53 ± 11.94 | 0.474 |
| Inter-group P-value b | - | 0.099 | 0.127 | - |
| HOMA | ||||
| Intervention | 5.82 ± 3.43 | 6.61 ± 4.27 | 4.14 ± 3.18 | < 0.001 |
| Control | 4.67 ± 3.01 | 4.38 ± 2.64 | 4.58 ± 2.66 | 0.737 |
| Inter-group P-value b | - | 0.025 | 0.275 | - |
a Independant samples t-test
b Paired samples t-test
5. Discussion
In this study, the effects of Urtica dioica L. in children with obesity were evaluated. We found that nettle can affect some metabolic variables such as FBS, TChol, LDL, and HOMA_IR significantly, and this demonstrates the potential effectiveness of herbal treatments in addition to conventional ones. Besides, there was no significant difference between the mean FBS in the control (P = 0.466) and the intervention groups (P = 0.331) in the three time periods.
In the study of Khajeh-Mehrizi et al., similar to our results, after 8 weeks, it was observed that nettle extract had no significant effect on FBS (22). Moreover, Mobaseri et al. found that glucose concentrations in human muscle cells did not change in the culture medium of nettle, which means that glucose was not taken up (26). Contrary to the results of our study, Namazi et al. found that after 8 weeks, the level of FBS and HBA1C showed a significant decrease, probably through pancreatic (increasing and improving the function of beta cells) or non-pancreatic mechanisms (decreased glucose transport from the small intestine) (27). Moreover, Fakhraee et al., assessed three possible mechanisms of the effects of nettle in lowering blood sugar. Its effect on muscle cells (GLUT4 coming to the membrane) and, as a result, increased glucose uptake, the pancreatic pathway (effect on beta, increasing insulin secretion, and ultimately reducing blood glucose), and carbohydrate hydrolysis (inhibition of alpha-amylase activity) were noticed (28). These possible mechanisms may be the reason for this inconsistency. Also, we have to consider different characteristics of the participants, such as older age and more frequent history of illness, or the diversity of taking nettle or its dosage.
Also, there was no significant difference in the mean weight in the intervention and control groups at the two investigated times (P = 0.330). However, Fan et al. observed that after 12 weeks, nettle reduced obesity through the mechanisms of fat accumulation and glucose metabolism in skeletal muscle, liver, and adipose tissue in mice (29). In a study by Madadi Jaberi et al., weight was significantly reduced in people who did not participate in any sports activities and only used nettle extract (30).
There was no significant difference between the mean blood TG level in the control group (P = 0.817) and the intervention group (P = 0.729) in the three periods. Contrary to ours, In Pashazadeh et al.'s study on mice, cholesterol and triglyceride levels decreased after 8 weeks (31). Farzami et al. also showed that nettle increases insulin secretion by stimulating the islets of Langerhans (32). But in the study of Ahangarpour et al., in rats, after a period of 8 weeks, nettle extract significantly increased TG (23). Although these different results may be regarding the studied population, which were animals, not humans, further studies are needed.
In the repeated measure of mean LDL in the intervention and control groups, it was found that the changing trend was significant and decreasing (P = 0.003) only for the intervention group over time. In the study of Daher et al., after 30 days, they reported a significant decrease in total cholesterol and LDL, which was in line with our study (33). Also, previous studies on animals showed that nettle extract reduced LDL significantly (23, 31). Regarding the consistency of the results, it seems that nettle can be a treatment option for patients with T1DM who have high LDL levels.
Furthermore, there were no significant differences in HDL cholesterol in control (P = 0.201) and intervention groups (P = 0.247) in the three investigated times, but in Namazi et al. HDL levels increased after receiving nettle for 8 weeks (27). Also, Pashazadeh et al. assessed mice and showed that HDL levels increased significantly after 8 weeks (31).
It was found that the mean total cholesterol was significant and decreased (P < 0.001) only in the intervention group. This can be because Nettle can reduce cholesterol serum concentration through the inhibitory effect of serotonin on the absorption, transfer, and metabolism of lipids (34). On the other hand, it seems that the saponin and tannin in nettle can reduce the intestinal absorption of fat and inhibit cholesterol esterase (which converts cholesteryl ester (a type of dietary lipid) into cholesterol and free fatty acid and finally reduce cholesterol (24).
Fasting insulin levels in the control group (P = 0.474) and the intervention group (P = 0.581) were not significantly different in the three investigated times. The present study’s findings are consistent with the findings of Riazi and Hassani. In their study, after intraperitoneal injection of nettle extract in mice, the insulin levels in the serum did not change, and nettle decreased blood sugar levels in mice by increasing insulin sensitivity (35).
Taking nettle can decrease IR and increase blood sugar entry into the cells, and, as a result, the stimulating effect of sugar is removed from the pancreas beta cells; consequently, insulin secretion decreases (24).
The trend of changes only for the intervention group during the time was increasing and then decreasing and was significant (P < 0.001). Consistent with the results of our study, in the study of Obanda et al. examining the effect of nettle on the skeletal muscle of mice, it was concluded that nettle improved glucose homeostasis and reduced IR caused by obesity through protein kinase B phosphorylation mechanism (36). In the study of Khajeh-Mehrizi et al., similar to our study, it was observed that the consumption of nettle extract reduced IR after 8 weeks (22). In addition, the IR index was reduced in the study of Ahangarpour et al. on rats after 8 weeks of nettle extract administration (23). Ranjbari et al. observed that serum glucose concentration decreased and insulin sensitivity increased significantly in the treated mice (21). Some compounds in nettle, including flavonoid, carotenoid, histamine, serotonin, acetylcholine, polysaccharide, and saponin, can increase insulin sensitivity (24) and consequently induce decreased IR.
5.1. Strength and Limitations
Despite the novelty of our study in children, it had some limitations. Our sample was children and adolescents aged 12-18 years, and we faced the difficulty of controlling their adherence to diet and medication. The subjects were of school-going age and were dealing with exams and the accompanying stress, potentially impacting their blood sugar levels. Also, exercising less or more and consuming less or more calories than required and usual by the patients may affect their fasting sugar, which could not be investigated.
5.2. Conclusions
This study revealed that administering nettle syrup could be helpful for patients with obesity with no significant adverse effects, and it considerably reduced LDL, Tchol, and HOMA-IR. Despite our promising results, it is recommended that further multicenter studies with larger sample sizes in this age group should be conducted to confirm our results.