1. Background
Celiac disease (CD) is a systemic immune disorder triggered by the ingestion of gluten and related prolamins found in barley, wheat, and rye. It occurs in genetically predisposed individuals and is characterized by a diverse range of gluten-dependent clinical symptoms and CD-specific antibodies, namely human leukocyte antigen-DQ8 (HLA-DQ8) or HLA-DQ2 and enteropathy (1, 2). Among these CD-specific antibodies are autoantibodies to transglutaminase 2 (TG2), including endomysial and deamidated gliadin peptide antibodies (3). The prevalence of biopsy-confirmed CD is estimated to be approximately 1% (4, 5). In Iran, CD is a common disease (1%) because wheat is an important staple food for the Iranian population (6). CD is associated with a wide range of clinical manifestations and symptoms, including weight loss, chronic diarrhea, and abdominal distension (7). Chronic diarrhea (lasting for more than two weeks) is a typical symptom of CD in children. Atypical manifestations include developmental disorders, short stature, iron deficiency anemia, hepatitis, mood disorder, ataxia, epilepsy, constipation, vomiting, infertility, enamel hypoplasia, delayed secondary sex characteristics, etc. (8, 9). Since CD may be asymptomatic, a significant number of patients remain undiagnosed and are exposed to complications such as malignancy, osteoporosis, and infertility (7). Therefore, a gluten-free diet (GFD) is the treatment that can relieve clinical symptoms, decrease antibody levels, and recover gastrointestinal (GI) mucosa in patients with CD (10, 11).
A GFD is a highly effective life-long treatment that facilitates rapid clinical recovery in patients. Conversely, histological improvement may require 1 - 2 years (12, 13). In some patients diagnosed with a delayed response, recovery may take over 5 years after adherence to a GFD. Studies have shown that up to 95% of children diagnosed with CD who adhered to a GFD for 2 years exhibited no mucosal damage (14). Patients with CD who do not experience clinical improvement in signs or symptoms while adhering to a GFD are classified as "non-responders" (15). Stasi et al.’s research in Italy revealed that approximately 1 in 50 CD patients did not respond to GFD, and the incidence of non-responders indicated the need for further research to optimize the management of CD (16). Another study by Dewar et al. showed that inadequate adherence to a GFD was the most common cause of patients’ GFD non-response (17). Pulloi et al. suggested that despite prolonged treatment and strict dietary adherence, mild or moderate GI symptoms might persist in some patients (18). Similarly, according to Sansotta et al.’s study, children who adhere to a strict GFD have more severe GI and extraintestinal (EI) symptoms than adults. Therefore, early CD diagnosis and strict adherence to a GFD may help alleviate symptoms (19). Norström et al. found that adherence to a GFD improved all symptoms of CD except joint pain. Furthermore, early CD diagnosis was found to be a crucial factor in improving outcomes (20). Conversely, Rubio-Tapia et al. reported that a significant proportion of adults with CD did not experience mucosal recovery after treatment with a GFD (21). Nevertheless, Galli et al. demonstrated that complete histologic recovery occurred in 66% of adult patients with CD after one year of GFD (22). Pulido et al.’s study revealed gender differences in clinical features related to before diagnosis, after GFD recovery, and quality of life, with females experiencing more difficulties than males (18).
2. Objectives
Although many research studies have been conducted on CD in recent years, the underlying pathogenesis mechanisms remain incompletely comprehended. However, there is evidence that genetic or environmental factors are implicated in the immune response to CD (23). This study aimed to evaluate the prevalence of non-responsive CD in pediatric patients with CD to identify the underlying causes.
3. Methods
This descriptive cross-sectional study aimed to investigate the prevalence and cause of persistent symptoms experienced by CD patients undergoing a GFD in Sistan and Baluchestan province, southeastern Iran, between 2017 and 2018. The study sample was selected from all diagnosed CD patients receiving a GFD for 6 months, and newly diagnosed cases studied within one year. The inclusion criteria were patients adhering to a GFD for six months, newly diagnosed cases who had been followed up, and CD patients with less than 21 years of age. Exclusion criteria included unwillingness to participate in the study and reluctance to continue participation.
After explaining the research objectives, all individuals signed the written informed consent. Regarding the enrollment of children and adolescents, parental consent was mandatory. A pediatric gastroenterologist confirmed all endoscopic biopsy specimens for CD based on clinical symptoms, positive tissue transglutaminase (TTG) and immunoglobulin A (IgA) antibodies, and any previous small intestine biopsy available in the medical records. In the present study, data were collected using a researcher-made questionnaire. The questionnaire consisted of 2 parts: (1) Demographic characteristics, including gender, age, age of diagnosis, duration of illness, family income, and place of residence; and (2) persistent symptoms at the onset and during treatment, including GI symptoms (abdominal pain, diarrhea, nausea, bloating, vomiting) and EI symptoms (weakness, arthralgia, dermatitis, infertility, weight loss or weight gain). The administered questionnaire contained questions of a closed-ended nature (yes/no), specifically designed to elicit responses in the affirmative or negative form on the presence of certain signs and symptoms. It is noteworthy that a similar questionnaire was also completed during the patient's referral period. Notably, GI and EI symptoms were documented on a questionnaire at the onset and during treatment. It is worth mentioning that patients who were asymptomatic or had related disease pathologies, such as inflammatory bowel disease (IBD) or peptic ulcer disease (PUD), were excluded from the study. Non-responsive CD patients who experienced no clinical symptom recovery or recurrent symptoms while maintaining a GFD were identified. Furthermore, CD patients underwent endoscopic examination for pathological evaluation after maintaining a GFD for one year. The present study analyzed the data using SPSS 16. The analysis descriptive section relied on the frequency distribution tables. To address inferential questions, the Kolmogorov-Smirnov test statistic was initially used to test for the normal distribution. For normally distributed data, we utilized independent t-test, univariate t-test, and analysis of variance (ANOVA). In cases where data deviated from the normal distribution, the nonparametric Mann-Whitney and Kruskal-Wallis tests were used to compare mean scores and the chi-square test for qualitative variables. The chi-square test was also employed to compare frequency distribution. The study's significance level was set at P ≤ 0.05.
4. Results
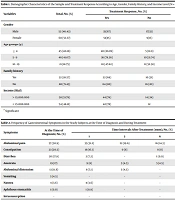
Between 2018 and 2019, a total of 199 patients referred to hospitals were diagnosed with CD, 87 of whom did not seek follow-up and assessment. Thus, a sample of 112 patients with CD undergoing a GFD was observed for one year, and their GI and EI symptoms were evaluated. The mean age of the patients was 82 ± 4.43 months, with an age range of 10 - 251 months. The demographic characteristics of the sample and their treatment response based on age, gender, family history, and income level were summarized in Table 1. At the end of the 1 year treatment, 26 patients (23.21%; 17 [33%] male, 9 [15%] female) reported experiencing at least one GI or EI symptom, indicating non-response. Table 2 presents the frequency of GI symptoms at the time of diagnosis and during treatment.
| Variables | Total, No. (%) | Treatment Response, No. (%) | P-Value | |
|---|---|---|---|---|
| Yes | No | |||
| Gender | ||||
| Male | 52 (46.43) | 35 (67) | 17 (33) | 0.027 a |
| Female | 60 (53.57) | 51 (85) | 9 (15) | |
| Age groups (y) | ||||
| ≤ 4 | 45 (40.18) | 40 (88.89) | 5 (11.11) | 0.001 a |
| 5 - 9 | 46 (41.07) | 36 (78.26) | 10 (21.74) | |
| 10 - 19 | 21 (18.75) | 10 (47.62) | 11 (52.38) | |
| Family history | ||||
| Yes | 32 (28.57) | 22 (69) | 10 (31) | 0.021 a |
| No | 80 (71.43) | 64 (80) | 16 (20) | |
| Income (Rial) | ||||
| > 15.000.000 | 58 (51.79) | 44 (76) | 14 (24) | 0.081 |
| < 15.000.000 | 54 (48.21) | 42 (78) | 12 | 22 |
Demographic Characteristics of the Sample and Treatment Response According to Age, Gender, Family History, and Income Level (N = 112)
| Symptoms | At the Time of Diagnosis; No. (%) | Time Intervals After Treatment (mo); No. (%) | |||
|---|---|---|---|---|---|
| 1 | 3 | 6 | 12 | ||
| Abdominal pain | 57 (50.9) | 35 (31.2) | 22 (19.6) | 16 (14.3) | 4 (3.6) |
| Constipation | 23 (20.5) | 18 (16.1) | 9 (8) | 9 (8) | 3 (2.7) |
| Diarrhea | 20 (17.9) | 2 (7.2) | - | 1 (0.9) | - |
| Anorexia | 19 (17) | 9 (8) | 5 (4.5) | 5 (4.5) | - |
| Abdominal distension | 13 (11.6) | 8 (7.1) | 5 (4.5) | - | - |
| Vomiting | 5 (4.5) | - | - | - | - |
| Nausea | 4 (3.6) | 4 (3.6) | - | - | - |
| Aphthous stomatitis | 1 (0.9) | 1 (0.9) | - | - | - |
| Intussusception | - | - | - | - | - |
Frequency of Gastrointestinal Symptoms in the Study Subjects at the Time of Diagnosis and During Treatment
As indicated in Table 2, all patients reported an improvement in vomiting a month after adhering to a GFD, and other GI symptoms also improved at 3-month and 6-month follow-ups. In addition, at the time of diagnosis and at the end of one year of treatment, symptoms of abdominal pain and constipation were reported more frequently.
Table 3 displays the frequency distribution of EI symptoms at the time of diagnosis and during treatment. The most common symptoms at the time of diagnosis and during treatment were weight loss and growth failure. As shown in Table 3, EI symptoms, including seizure, were not apparent one month after adhering to a GFD, and some non-GI symptoms were improved within 3, 6, and 12 months after treatment.
| Symptoms | At the Time of Diagnosis; No. (%) | Time Intervals After Treatment (Mon); No. (%) | |||
|---|---|---|---|---|---|
| 1 | 3 | 6 | 12 | ||
| Weight loss | 73 (65.2) | 63 (56.2) | 31 (27.7) | 19 (17) | 14 (12.5) |
| Growth failure | 60 (53.6) | 49 (43.8) | 25 (22.3) | 14 (12.5) | 11 (9.8) |
| Anemia | 35 (31.2) | 23 (20.5) | 12 (10.7) | 3 (2.7) | 1 (0.9) |
| Enamel hypoplasia | 19 (17) | 16 (14.5) | 12 (10.7) | 12 (10.7) | 7 (6.2) |
| Weakness | 14 (12.5) | 6 (5.4) | - | - | - |
| Arthralgia | 12 (10.7) | 10 (8.9) | 10 (8.9) | 10 (8.9) | 6 (5.4) |
| Rickets | 10 (8.9) | 9 (8) | 6 (5.4) | 2 (1.8) | - |
| Dermatitis | 8 (7.4) | 6 (5.4) | 2 (1.8) | 3 (2.7) | - |
| Seizure | 1 (0.9) | - | - | - | - |
| Alopecia | - | 1 (0.9) | - | - | - |
| Muscle atrophy | - | - | - | - | - |
| Maturity delay | - | - | - | - | - |
Frequency Distribution of Extraintestinal Symptoms During Treatment
At the end of a one-year follow-up, only 6 patients were satisfied with undergoing endoscopy and re-examination for histological and pathologic improvement. According to the pathological results obtained from 6 patients, 2 were normal, 3 had partial recovery, and one showed no different pathological result compared to the onset of the diagnosis (Table 4).
| Variables | Disease Onset | At the End of a One-Year Follow-up |
|---|---|---|
| Marsh classification | IIIc | IIIa |
| IIIc | IIIa | |
| IIIa | I | |
| I | I | |
| IIb | Normal | |
| IIIb | Normal |
Frequency of Pathologic Findings in Celiac Patients with Stable Symptoms on a Gluten-Free Diet at the Time of Diagnosis and at the End of a One-Year Follow-up
5. Discussion
According to the results of the present study, the most common GI symptoms at the time of diagnosis were abdominal pain with a frequency of 50.9%, followed by constipation with a frequency of 20.5%. Weight loss and growth failure were the most common EI symptoms of CD patients, with a frequency of 65.2% and 53.6%, respectively. These findings align with various investigations; for instance, Sansotta et al. reported that adults experienced abdominal pain, diarrhea, and bloating the most, while children had abdominal pain, diarrhea, and developmental disorders as the most frequent symptoms (19). However, in another study, Pulido et al. indicated bloating and abdominal pain (84.9%), tiredness and extreme weakness (74.2%), anemia (67.8%), and diarrhea (71.7%) as the most common symptoms during diagnosis, which do not concur with our findings (18). Regarding EI-related symptoms, psychiatric disorders, iron deficiency anemia, headache, and fatigue were observed frequently in adults, while short stature, headache, and fatigue were the most common in children, as noted by a previous study (19). However, our study revealed that convulsions and other non-GI symptoms were not evident after one month of adhering to a GFD. Moreover, these symptoms significantly improved within 3, 6, and 12 months after treatment. After a year of assessment, we found that enamel hypoplasia (6.2%), arthralgia (5.4%), and anemia (0.9%) were the most common non-GI symptoms that did not respond to treatment.
Laurikka et al.’s study on GI symptoms in patients with CD adhering to a long-term GFD found that untreated CD patients experienced more indigestion, diarrhea, and abdominal pain than controls and the groups adhering a GFD. Moreover, CD patients who received treatment for more than 10 years reported more reflux, while those treated for 1 - 2 years had more diarrhea than controls. Long-term treated CD patients exhibited relatively mild symptoms compared to other GI diseases (24). Sansotta et al. also reported that children adhering to a strict GFD exhibited higher rates of both EI and GI symptoms remission than adults, with greater rates of recovery in GI over EI symptoms. Early CD diagnosis and strict adherence to a GFD may aid in remission (19), which aligns with our findings. Murray et al. reported that although diarrhea was the most prevalent symptom in untreated CD patients, steatorrhea occurred in only one-fifth of them. Other complaints were prevalent and mostly responded to the GFD. The efficacy of GFD was observed to be equal in both genders (25).
Regarding the pathological results obtained from the present study, it was observed that the majority of patients exhibited either normal or partial recovery. Interestingly, one patient displayed no significant deviation from the pathological results at the onset of diagnosis. It is noteworthy that Rubio-Tapia et al. have also conducted research in this area and have reported that a considerable number of adult patients with CD exhibit no improvement in mucosal health after undergoing treatment with a GFD (21). The only discrepancy was related to the age of the sample population, indicating that mucosal response and endoscopic examination are weaker in children than in adults adhering to a GFD, which can be attributed to the chronic trend of gluten contact in the adult intestine. Galli et al. have also reported that after one year of adhering to a GFD, 66% of adult patients with CD showed complete histological improvement (22).
Our findings have demonstrated no significant correlation between income level and the rate of treatment non-response in patients. Additionally, it has been observed that patients with a positive family history exhibit a significantly higher rate of non-response than those with a negative family history. This outcome is consistent with the findings of Stasi et al.’s (16) study, reporting that 21.8% of patients experienced recurrent or persistent symptoms. Moreover, 23% of patients showed positive results for anti-endomysial antibodies. In patients diagnosed with CD, gluten exposure was found to be the primary cause of recurrent signs/symptoms, and a modified diet resulted in remission in 63% of the patients during later follow-up periods. Similarly, Dewar’s study analyzed a total of 112 consecutive patients referring for non-responsive CD, wherein 9% were diagnosed with refractory CD after two years. Furthermore, their findings indicated that inadequate adherence to a GFD was the primary reason for non-responders (17), which is in line with the results of the present study.
5.1. Conclusions
The present study revealed that the predominant symptoms observed during the diagnosis were weight loss followed by growth failure. The male population had a higher incidence of non-response than their female counterparts. After one year of treatment, weight loss, growth failure, abdominal pain, and constipation were the prevailing symptoms that exhibited resistance to EI and GI therapy. Consequently, the findings suggest that GFD has a noteworthy impact on reducing GI and EI symptoms; however, its effect on persistent symptoms, particularly among old patients, is negligible.