1. Background
The coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, has resulted in lung failure and the need for mechanical ventilation (1-3). Initially, during the early stages of the COVID-19 pandemic, the incidence of positive cases among children was deemed negligible, and it was assumed that children were less susceptible (4-6). However, subsequent studies have indicated that children and adolescents are also vulnerable to SARS-CoV-2 infection. Consequently, since March 2020, over 7 million children in the United States have been confirmed to be positive for the virus (7, 8). Notably, children with COVID-19 have shown milder symptoms and a lower risk of hospitalization and mortality compared to adults (9). A recent systematic review reported that children with COVID-19 may experience symptoms such as fever, respiratory issues, and gastrointestinal symptoms (10).
As the full scope of COVID-19 remains unknown, and there is no definitive treatment for children, various drugs such as remdesivir, systemic corticosteroids, and intravenous immunoglobulin have been utilized in hospitalized children, and several clinical trials have yielded diverse outcomes (11). Since the 1960s, N-acetylcysteine (NAC) has been used for its mucolytic effect in patients with chronic bronchitis, cystic fibrosis, and bronchiectasis (12), as well as for acute liver failure and acetaminophen overdose (13, 14). The safety of NAC, even up to 2700 mg, has been well established in previous studies (15), and its efficacy in lung disorders extends beyond its mucolytic activity. It operates through various mechanisms, such as enhancing the immune system, inhibiting viral replication, and reducing inflammation by increasing intracellular cysteine storage (16-18).
2. Objectives
Despite its safety and potential benefits for respiratory diseases, evidence of its use in children with COVID-19 was lacking. Therefore, we aimed to analyze whether NAC could improve outcomes in hospitalized children with respiratory symptoms caused by COVID-19 through a double-blind, placebo-controlled, randomized clinical trial. We hypothesized that administering NAC could be a suitable treatment option for these children.
3. Methods
In this double-blinded randomized controlled trial, we evaluated the impact of administering 600 mg of NAC twice daily on the prognosis of hospitalized children with PCR-confirmed moderate COVID-19. Eligible patients were selected from the infectious department of the 17 Shahrivar Hospital in Rasht, Iran. Prior to enrollment, written informed consent was obtained from the parents or guardians of the patients. The trial protocol received approval from the Ethical Committee of Guilan University of Medical Sciences (code: IR.GUMS.REC.1401.018) and was also registered with the Iranian Clinical Trial Registry (IRCT20220516054879N1).
As a pilot study, the sample size comprised 58 patients divided into two groups. The inclusion criteria were ages between 8 to 18 and a definitive diagnosis of moderate COVID-19 infection confirmed by PCR, alongside evidence of lower respiratory disease through clinical assessment or imaging, and patients with an oxygen saturation (SpO2) of ≥ 94% on room air at sea level.
Exclusion criteria included patients with mild disease lacking shortness of breath, dyspnea on exertion, or abnormal imaging; severe disease requiring ICU care, SpO2 < 94% on room air at sea level, PaO2/FiO2 < 300 mm Hg, a respiratory rate > 30 breaths/min, or lung infiltrates > 50% (19); patients who were immunosuppressed or receiving chronic immunosuppressive therapy (equivalent daily dose of ≥ 20 mg prednisolone for > 2 weeks), had asthma, a history of bronchospasm, or a known hypersensitivity reaction to NAC.
Patients were randomly divided into either the NAC group or the placebo group (the placebo contained polyvinyl pyrrolidone, sodium saccharin, sodium bicarbonate, and lactose monohydrate). The NAC group received the national protocol treatment for COVID-19 plus NAC (1200 mg/day) at a dose of 600 mg twice daily. The non-NAC group received routine treatment plus a placebo. The drugs and placebos, provided by OSVE Pharmaceutical Company (Tehran, Iran), were administered orally for 7 days. Both groups were monitored at the start of the study and after 7 days for the incidence of NAC-related side effects, drug interactions, C-reactive protein (CRP) levels, white blood cell (WBC) count, serum creatinine, oxygen saturation, hospital stay duration, and clinical symptoms.
The primary outcomes of this study were the improvement of clinical signs and symptoms and oxygen saturation levels. Secondary outcomes included the duration of hospital stay, mortality rate, and adverse effects. Patients were randomly assigned to either the NAC group or the placebo group using permuted block randomization. Participants and caregivers, as well as radiologists, researchers evaluating the outcomes, and the statistician, were blinded in this study.
Data analysis was conducted using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). A P-value of less than 0.05 was deemed significant. Group comparisons were made using independent samples t-tests, the Mann-Whitney test, and the chi-square test.
4. Results
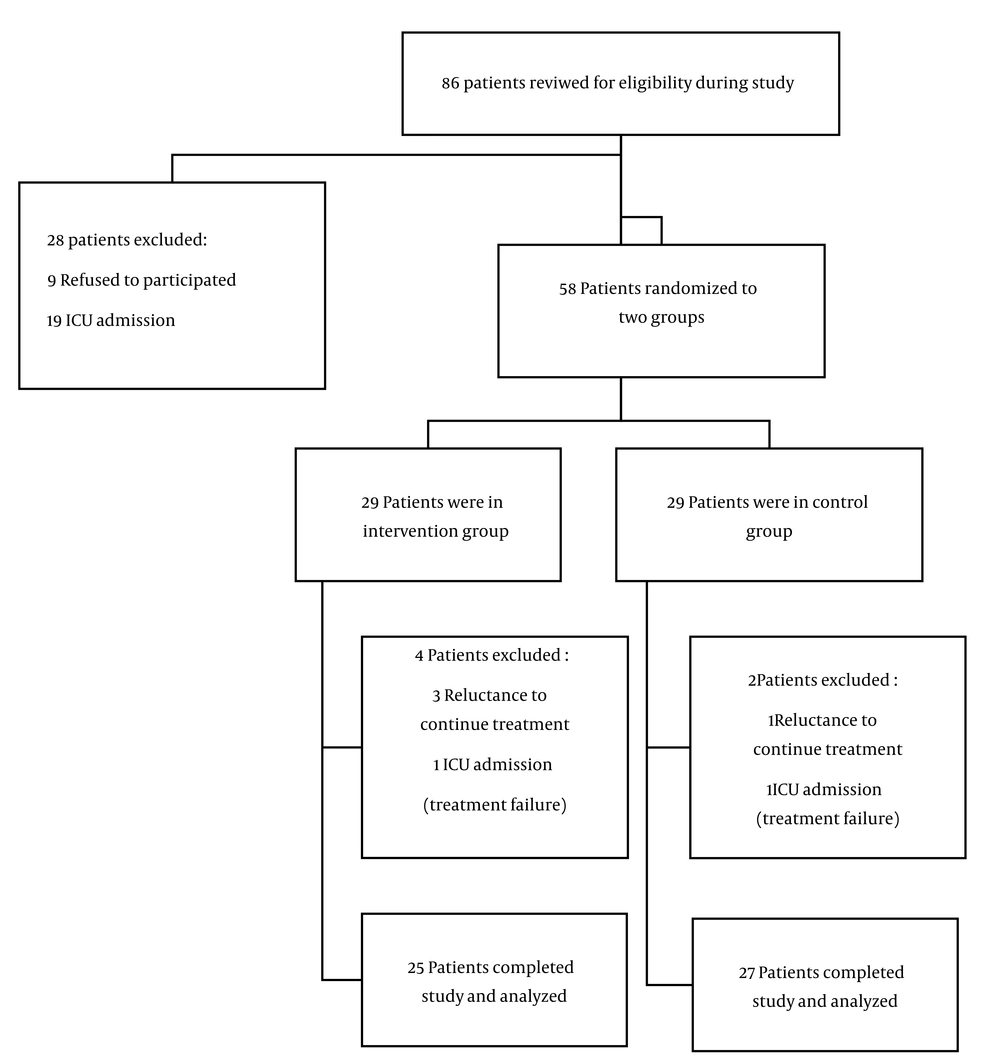
From June 2022 to December 2022, 86 patients with a positive PCR test for COVID-19 were admitted to our hospital. Of these, 28 were excluded, leaving 58 patients to be enrolled in the study. Six patients were later excluded due to either an unwillingness to continue with the drug or the need for ICU admission, resulting in 52 patients completing the study and being included in the analysis.
The CONSORT flow diagram is depicted in Figure 1.
Table 1 presents the demographic characteristics and clinical variables, showing no significant differences in demographic data, past medical history, or clinical signs and symptoms (such as fever, myalgia, dyspnea, cough, gastrointestinal symptoms, oxygen saturation, WBC, and CRP between the groups at the start of the study.
| Variables | Intervention Group (n = 25) | Control Group (n = 27) | P-Value |
|---|---|---|---|
| Female/male | 13/12 | 15/12 | 0.797 |
| Age, y | 10.8 ± 2.72 | 11.33 ± 2.18 | 0.436 |
| Weight, kg | 36.88 ± 14.35 | 42.14 ± 19.31 | 0.273 |
| White blood cell, × 109/L | 10.00 (8.50 -11.70) | 11.00 (7.00 - 14.00) | 0.091 |
| Oxygen saturation, % | 86.36 ± 5.04 | 89.25 ± 4.84 | 0.251 |
| Baseline CRP, mg/L | 12.00 (9.50 - 20.00) | 14.00 (6.40 -25.00) | 0.065 |
| Time from starting symptoms to start of interventions, day | 3.24 ± 2.22 | 5.00 ± 4.50 | 0.084 |
a Values are expressed as mean ± SD or median (IQR).
By the end of the intervention, all investigated variables in both groups had significantly improved from the beginning of hospitalization, as detailed in Table 2.
| Variables | Intervention Group (n = 25) | Control Group (n = 27) | ||||
|---|---|---|---|---|---|---|
| Baseline | After 7 Days | P-Value | Baseline | After 7 Days | P-Value | |
| Serum creatinine, mg/dL | 0.80 (0.60 - 0.85) | 0.50 (0.50 - 0.65) | < 0.001 | 0.90 (0.80 - 1.00) | 0.70 (0.60 - 0.70) | < 0.001 |
| White blood cell, × 109/L | 10.00 (8.5 - 11.70) | 7.45 (5.50 - 6.10) | < 0.001 | 11.00 (7.00 - 14.00) | 7.50 (5.80 - 10.40) | 0.070 |
| Oxygen saturation, % | 86.36 ± 5.04 | 98.84 ± 0.98 | < 0.001 | 89.25 ± 4.84 | 96.81 ± 5.12 | < 0.001 |
| C-reactive protein, mg/L | 9.50 (12.00 - 20.00) | 4.00 (2.50 - 9.00) | < 0.001 | 14.00 (6.40 - 25.00) | 5.00 (1.10 - 10.00) | < 0.001 |
a Values are expressed as mean ± SD or median (IQR).
Table 2 also outlines the changes in CRP levels between the groups, revealing no significant difference at the start (P = 0.065) or the end of the study (P = 0.659).
Similarly, changes in WBC counts, as shown in Table 2, indicated no significant difference at the study's outset or after 7 days between the two groups (P = 0.091 and 0.067, respectively).
Table 2 further demonstrates that, while there was no significant difference in oxygen saturation between the two groups at baseline (P = 0.251), a significant improvement was noted in the NAC group compared to the placebo group by the study's end (P = 0.001).
According to Table 3, the duration of hospital stay was significantly different between the intervention and placebo groups (P-value = 0.001). The mortality rates between the intervention and placebo groups did not significantly differ (P-value = 0.491).
| Variables | Intervention Group (n = 15) | Control Group (n = 15) | P-Value |
|---|---|---|---|
| Hospitalization | 6.24 ± 2.75 | 11.20 ± 6.16 | 0.001 |
| Mortality | 0/25 (0) | 2/27 (7.4) | 0.491 |
a Values are expressed as mean ± SD or No. (%).
Both NAC and the placebo were well tolerated, with only one patient in the placebo group reporting a rash that was not severe and did not necessitate stopping the treatment.
5. Discussion
Since 2019, various drugs have been tested for the treatment of COVID-19. N-acetylcysteine has been utilized as a mucolytic agent (20, 21) that reduces inflammation (22), restores the activity of the intracellular antioxidant system (23-25), and modulates the immune system (26). While several studies have reported significant benefits of NAC in treating severe COVID-19 infection or other acute respiratory symptoms in adults, to our knowledge, our clinical trial is the first to evaluate the efficacy and safety of NAC in children with moderate COVID-19.
This double-blind, placebo-controlled, randomized trial involved 58 children diagnosed with moderate COVID-19. At the outset, there were no significant differences in clinical signs and symptoms between the NAC and placebo groups. At the study's conclusion, the WBC and CRP levels showed no significant differences between the groups (P = 0.069 and P = 0.067, respectively). This outcome aligns with the findings of Rahimi et al. (27) and contrasts with Ibrahim et al., where a decrease in CRP levels was observed (28). The discrepancies between these studies might be attributed to the non-specific nature of CRP and WBC in solely evaluating COVID-19. Additionally, the administered dose (600 mg twice daily via oral route) may not have been potent enough to elicit a positive response in non-selective inflammatory markers.
In our study, NAC significantly reduced serum creatinine levels (P = 0.001), echoing previous studies that demonstrated a significant reduction in serum creatinine following NAC administration (29, 30). Furthermore, the NAC group exhibited better oxygen saturation levels than the placebo group at the study's end (P = 0.001), a finding consistent with Panahi et al., who noted significant improvements in oxygen saturation and more rapid lung damage reduction in COVID-19 patients treated with NAC compared to a control group (31).
N-acetylcysteine supplementation also resulted in a shorter hospital stay, corroborating other studies that have linked NAC administration with reduced hospitalization duration. Bhattacharya et al. reported a decrease in hospital stays and an increase in hospital discharge rates with NAC administration (32). Jorge-Aarón et al. observed positive outcomes of NAC as an adjuvant treatment in COVID-19 patients, leading to reduced hospitalization time (24).
Our results, however, did not demonstrate an increase in patient survival rates with NAC treatment. This finding contrasts with Bhattacharya et al., who reported a reduction in mortality among COVID-19 patients treated with NAC without significant adverse effects (32). Meanwhile, Taher et al. found no link between NAC administration and mortality rates (33).
There are several potential justifications based on the limitations of these results. First, a significant proportion of patients in both groups in our study received corticosteroids, suggesting that corticosteroids, acting as a potent anti-inflammatory treatment in COVID-19 patients, might negate any additional effects from NAC. Second, the small sample size of our study means we could only detect large effects. Third, although the use of other COVID-19 treatments did not vary between the two groups, the permitted use of these medications might have influenced the effects of NAC on the clinical outcomes of the study populations. Fourth, more specific inflammatory markers such as interleukins, total antioxidant capacity, and tumor necrosis factor-alpha were not evaluated, leading to ambiguous correlations between the effect of NAC on inflammatory parameters and the clinical status of the patients. Fifth, our study was conducted at a single center. Therefore, drawing broad conclusions about the efficacy of NAC as adjunctive therapy in patients with COVID-19 from the results of a single-center study is impractical. Larger, multicenter studies are necessary to ascertain its potential efficacy in such patients.
5.1. Conclusions
In conclusion, the findings of this study support the notion that NAC could be beneficial in reducing the length of hospital stay and improving oxygen saturation in children with COVID-19.