1. Background
Familial Mediterranean fever (FMF) is an autosomal recessive disease characterized by recurrent episodes of fever and symptoms of serous inflammation, ultimately leading to abdominal, joint, and chest pain (1, 2). It is most commonly observed in populations living in the Mediterranean region, including Jews, Arabs, Turks, and Armenians (1). The prevalence of FMF in the Middle East ranges from approximately 1 in 2000 to 1 in 100 (3). Diagnosis of FMF is primarily clinical, and one of the most significant complications is amyloidosis, which can result in kidney failure (4). Its symptoms usually manifest in childhood or adolescence in over 80% of patients (5). Colchicine, an alkaloid derivative with anti-inflammatory effects, is the only known drug that can decrease the incidence and severity of disease episodes and prevent the development of amyloidosis (6). Studies have shown that the genetic basis of FMF is linked to mutations in the MEFV gene, located on the short arm of chromosome 16 at position 16P13.3 (7). The M694V gene mutation is the most prevalent among FMF patients (8). This mutation is associated with an earlier onset of the disease, a higher incidence of arthritis, and an increased risk of amyloidosis (9). Identifying mutations associated with this disease can significantly aid in early diagnosis and the prevention of affected births (10). Since FMF is relatively prevalent in the Mediterranean region, most of the published research on this disease has been conducted in this area. A 2014 study conducted in Ardabil reported that the genetic profile of Iranian FMF patients is similar to that of Armenians and Arabs, with the M694V and V726A gene mutations being the most common among patients (11). However, only a few studies have explored the genetic and clinical profile of FMF in eastern Iran.
2. Objectives
This study aims to investigate the frequency, genetic, and clinical features of children with FMF in northeastern Iran.
3. Methods
3.1. Study Design and Participants
Our study was a descriptive and analytical cross-sectional study conducted among 29 patients under the age of 18 who visited the pediatric Rheumatology Department at Akbar Children's Subspecialty Hospital, a referral center in northeastern Iran, between April 2014 and April 2021. The inclusion criteria for the study were patients under 18 years of age with a confirmed diagnosis of FMF by a pediatric rheumatology subspecialist or approved by a genetic counselor. For the clinical diagnosis of FMF, we used Tel-Hashomer criteria, requiring at least 2 major criteria or 1 major and 2 minor criteria for diagnosis. The major criteria include fever with peritonitis, synovitis or pleurisy, recurrent febrile attacks, and AA amyloidosis. The minor criteria include erysipeloid erythema, response to colchicine, and family history of the periodic syndrome. For further details and explanations, please refer to (12). Exclusion criteria included parents not consenting to participate in the study, failure to attend the children's rheumatology clinic regularly for follow-up, arbitrary discontinuation of medication without coordination with the attending physician, lack of cooperation and participation of patients in providing information through phone calls, and incomplete medical records of patients.
3.2. Data Gathering
After the clinical diagnosis, the parents were provided with an explanation of the situation, and the patients were subsequently referred for genetic evaluation. This study focused on patients who had received a definitive diagnosis of FMF and were being treated with drugs such as colchicine and dapsone. DNA extraction was performed using 3 mL of whole blood following the salting-out DNA extraction procedure. All exons of the MEFV gene were amplified using specific primers for each exon (Table 1). Sanger sequencing was conducted using an ABI 3130xl sequencer according to the manufacturer's instructions. Data were analyzed using the sequencer software, and the pathogenicity of candidate variants was assessed following the American College of Medical Genetics and Genomics (ACMG) guidelines, utilizing bioinformatics prediction tools such as Mutation Taster (http://www.mutationtaster.org), SIFT (sift.jcvi.org), ClinVar (https://www.ncbi.nlm.nih.gov/clinvar), and PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2). Sequencing results underwent a quality evaluation. In addition to the internal analysis performed by the sequencing equipment's software, a quality analysis review of the sequencing data was carried out by visually inspecting both strands. The data used in our study were obtained from the gold standard repositories mentioned above, indicating that data quality had already been assessed. Both forward and reverse sequencing were conducted to confirm detected mutations.
| Variables | Primers Sequence | Product Size |
|---|---|---|
| Exon 1 | 473 | |
| FMF-1F | TTCCCACCAAGACACAGAGC | |
| FMF-1R | CTTTCCCACAAAGCAGCCAG | |
| Exon 2 | 739 | |
| FMF-2F | ATCTTGGGCCCTAAACGTGG | |
| FMF-2R | GCCATTCTTTCTCTGCAGCC | |
| Exon 3 | 469 | |
| FMF-3F | CCGCTGTGCTTTGTGATACC | |
| FMF-3R | AAGTGCCTGGCAGAGAAGAG | |
| Exon 4 | 239 | |
| FMF-4F | CAGCTAAAGATGGCAGGAGC | |
| FMF-4R | CTGCTGGTTACCCTCTGTCC | |
| Exon 5 | 355 | |
| FMF-5F | GGTTCCTGGACATCCACGTC | |
| FMF-5R | CCTGAGGCATCCTGATAGGC | |
| Exon 6 | 254 | |
| FMF-6F | GCCACTAGGAGCCTGGTAAC | |
| FMF-6R | CTGACCAGATGCCCTTCTCC | |
| Exon 7-8-9 | 852 | |
| FMF-7-8-9F | TTCCAGCTCACGGGTACTTG | |
| FMF-7-8-9R | AGAGCACAGGGATCCAGCAG | |
| Exon 10 | 839 | |
| FMF-10F | CCTGTCCCTGTTTCCTGCAG | |
| FMF-10R | ATGTCTTCACCCGGATTGAC |
The variables considered in this study included demographic information such as patient age, age of disease onset, sex, height, weight, parental history, disease duration, rate of hospitalization, and clinical symptoms (fever, abdominal pain, chest pain). Laboratory tests conducted during attacks (erythrocyte sedimentation rate [ESR], C-reactive protein [CRP], white blood cells [WBC]) and complications (amyloidosis and intestinal obstruction) were also recorded, along with details of drug treatment and dosage (colchicine, dapsone, etc.). Clinical follow-up of patients was conducted through telephone calls and regular visits. The study identified mutations related to each patient and calculated the frequency of each mutation using SPSS v. 16 software (SPSS Inc., Chicago, IL, USA) to analyze the data extracted from the checklists.
3.3. Statistical Analysis
The data were analyzed using SPSS v. 16. Mean ± standard deviation (SD) was calculated for quantitative variables, and frequencies and percentages were reported to describe qualitative variables. Specifically, the frequency, percent, and distributional measures were added. A cross-sectional analysis was conducted to classify variables into groups as benign, variant of uncertain significance (VUS), and pathogenic. Furthermore, analysis of variance (ANOVA), including mean differences and P-values, was conducted to compare group means for a significant statistical analysis. Multiple comparisons were also included to specify variables with equal group means.
3.4. Ethical Considerations
This study was approved by the Vice-Chancellor of Research at Mashhad University of Medical Sciences (code: IR.MUMS.MEDICAL.REC.1401.112). Written informed consent letters were obtained from all parents/guardians.
4. Results
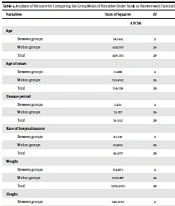
This study included 29 patients with FMF, consisting of 17 girls (58.6%) and 12 boys (41.4%). The average age of the patients was 9.34 ± 4.27 years, with the youngest being 3 years old and the oldest 18. The average age of disease onset was 3.29 ± 2.54 years, with the youngest starting at 1 year and the oldest at 13 years. Among the 17 female patients, 35.3%, 17.6%, and 47% were categorized as benign, VUS, and pathogenic, respectively. Similarly, among the 12 male patients, 75%, 8.3%, and 16.6% were categorized as benign, VUS, and pathogenic, respectively. Overall, one-third (34.5%) of the studied patients were classified as pathogenic. Furthermore, 48.3% of the patients had parents who were related to each other. The majority of patients (79.3%) had FMF for more than 24 months. All patients had experienced at least 3 bouts of fever before participating in the study, and 51.7% had abdominal pain. However, other symptoms, such as rash and myalgia, were not observed among the patients. Arthritis was present in 13 patients (44.8%), with most cases involving the knee (37.9%). In terms of laboratory parameters, all patients had a WBC count higher than 15 000 (with an average of 18 000.9 ± 0.62 mcL), and during acute attacks, ESR (with an average of 64.10 ± 3.17 mm) and CRP (with an average of 59.82 ± 4.51 mg/L) levels were elevated. Refer to Table 2 for a more detailed statistical analysis. Colchicine was used by 28 patients (96.6%). Regarding colchicine dosage and the treatment plan, patients were treated with a standard dose of colchicine. Young children were treated with 0.3 mg/kg, while pediatric patients received the same dose as adults, which is 1 - 2 mg/kg. Furthermore, none of the patients had comorbidities, and a few patients reported abdominal discomfort and diarrhea, which were managed by slowly titrating the drug. In total, 97% of patients responded to treatment. One patient with symptoms of pleural and pericardial effusion, dyspnea, and chest pain did not respond to colchicine and required dapsone for symptom control and to manage FMF attacks. In terms of patient zygosity, 23 patients (79.3%) were heterozygous, and 6 patients (20.6%) were homozygous (see Table 3). The classification of MEFV gene mutations showed that four patients (13.8%) had VUS variants, 10 patients (34.5%) had pathogenic mutations, and 15 patients (51.7%) had benign variants. The most common amino acid substitution among patients was P. Glu148Gln (E148Q), which was reported as benign, with a frequency of 41.3%, followed by pathogenic mutations P. Met680Ile (M680I), with a frequency of 10.2%. The most common nucleic acid alteration in this study was c.442G>C, with an abundance of 41.3%. Clinical diagnosis suggested FMF, as mutations in the MEFV gene are associated with this disease. To identify the causative mutation, we sequenced all ten exons of the MEFV gene with specific primers using the Sanger sequencing method performed by the ABI 3130xl sequencer according to the manufacturer's instructions. Data were analyzed using sequencer software, and the pathogenicity of candidate variants was evaluated using the VarSome tool following the ACMG guidelines. The results are summarized in Table 1. For significant statistical analysis, we compared the means of age, age of onset, disease duration, rate of hospitalization, weight, and height using ANOVA at the significance level α = 0.05. If μbenign, μvus, and μpathogen denote the mean of any variables mentioned above for the 3 groups, i.e., benign, VUS, and pathogenic, respectively, then using P-values, we observed that for all variables except for the rate of hospitalization (with a P-value equal to 0.037), the null hypothesis Ho: μbenign = μvus = μpathogen was accepted at the 0.05 significance level, indicating no significant difference between these groups. For the rate of hospitalization, the null hypothesis Ho was accepted, and consequently, a post-hoc test was conducted to determine which groups had different means for this variable. Accordingly, the 2 groups, benign and pathogenic, had different means, with a P-value of 0.039 in the multiple comparisons based on the Tukey HSD (Tables 2 - 8 show the detailed results).
| Variables | Mean ± SD | Min | Max |
|---|---|---|---|
| Age | 9.34 ± 4.27 | 3 | 18 |
| Height | 130 ± 20 | 95 | 171 |
| Weight | 30 ± 13.7 | 14 | 65 |
| Attack WBC (mcL) | 18000.9 ± 0.62 | 15000 | 28000 |
| Attack ESR (mm/h) | 64.10 ± 3.17 | 37 | 95 |
| Attack CRP (mg.L) | 59.82 ± 4.51 | 20 | 105 |
| Variables | Frequency | Percent (%) | Cumulative P |
| Sex | |||
| Male | 13 | 44.8 | 100 |
| Female | 16 | 55.2 | 55.2 |
| Consanguinity of parents | |||
| Yes | 15 | 51.7 | 51.7 |
| No | 14 | 48.3 | 100 |
| Family history | |||
| None | 25 | 86.2 | 86.2 |
| FMF | 2 | 6.9 | 93.1 |
| PFAPA | 2 | 6.9 | 100 |
| Colchicine | |||
| Yes | 28 | 3.4 | 3.4 |
| No | 1 | 96.6 | 100 |
Abbreviations: WBC, white blood cells; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein.
| Mutations and Genotypes | Number of Patients (%) |
|---|---|
| Heterozygote; N = 23 (79.3%) | |
| A744S | 2 (6.8) |
| R408Q | 2 (6.8) |
| R761H | 1 (3.4) |
| E148Q | 9 (31) |
| E148V | 2 (6.8) |
| M680I | 2 (6.8) |
| M694I | 1 (3.4) |
| P369S | 1 (3.4) |
| V726A | 1 (3.4) |
| E148D | 2 (6.8) |
| Homozygote; N = 6 (20.6%) | |
| E148Q | 3 (10.3) |
| M694V | 2 (6.8) |
| M680I | 1 (3.4) |
| Clinical Features | Number of Patients (%) |
|---|---|
| Fever | 29 (100) |
| Abdominal pain | 15 (51.7) |
| Chest pain | 2 (6.9) |
| Arthritis | 13 (44.8) |
| Pruritus | 1 (3.4) |
| Rash (Erysipelas-like erythema) | 0 (0) |
| Myalgia | 0 (0) |
| Numbers | Variant | Amino Acid | Classification | No. (%) |
|---|---|---|---|---|
| 1 | c.2230G>T | A744S | Likely Benign | 2 (6.8) |
| 2 | c.442G>C | E148Q | Benign | 12 (41.3) |
| 3 | c.1105C>T | P369S | Benign | 1 (3.4) |
| 4 | c.1223G>A | R408Q | VUS | 2 (6.8) |
| 5 | c.443A>T | E148V | likely pathogenic | 2 (6.8) |
| 6 | c.2177T>C | V726A | Likely pathogenic | 1 (3.4) |
| 7 | c.2282G>A | R761H | Pathogenic | 1 (3.4) |
| 8 | c.2082G>A | M694I | Pathogenic | 1 (3.4) |
| 9 | c.2080A>G | M694V | Pathogenic | 2 (6.8) |
| 10 | c.2040G>C | M680I | Pathogenic | 2 (6.8) |
| 11 | c.2040G>A | M680I | Pathogenic | 1 (3.4) |
| 12 | c.444G>C | E148D | VUS | 2 (6.8) |
Abbreviation: VUS, variant of uncertain significance
a The frequency and percentage of nucleic acid types in patients with familial Mediterranean fever participating in the study are denoted by No. (%).
| Crosstab | |||
|---|---|---|---|
| Classifications | Sex | Total | |
| Female | Male | ||
| Benign | |||
| Count | 6 | 9 | 15 |
| % Within classification | 40.0 | 60.0 | 100.0 |
| % Within sex | 35.0 | 75.0 | 51.7 |
| VUS (variant of uncertain significance) | |||
| Count | 3 | 1 | 4 |
| % Within classification | 75.0 | 25.0 | 100.0 |
| % Within sex | 17.6 | 8.3 | 13.8 |
| Pathogen | |||
| Count | 8 | 2 | 10 |
| % Within classification | 80.0 | 20.0 | 100.0 |
| % Within sex | 47 | 16.6 | 34.5 |
| Total | |||
| Count | 17 | 12 | 29 |
| % Within classification | 58.6 | 41.4 | 100.0 |
| % Within sex | 100.0 | 100.0 | 100.0 |
| ANOVA | |||||
|---|---|---|---|---|---|
| Variables | Sum of Squares | df | Mean Square | F | P-Value |
| Age | 0.435 | 0.652 | |||
| Between groups | 14.544 | 2 | 7.272 | ||
| Within groups | 434.767 | 26 | 16.722 | ||
| Total | 449.310 | 28 | |||
| Age of onset | 0.024 | 0.976 | |||
| Between groups | 0.288 | 2 | 0.144 | ||
| Within groups | 155.850 | 26 | 5.994 | ||
| Total | 156.138 | 28 | |||
| Disease period | 1.048 | 0.365 | |||
| Between groups | 1.235 | 2 | 0.618 | ||
| Within groups | 15.317 | 26 | 0.589 | ||
| Total | 16.552 | 28 | |||
| Rate of hospitalization | 3.756 | 0.037 | |||
| Between groups | 10.357 | 2 | 5.178 | ||
| Within groups | 35.850 | 26 | 1.379 | ||
| Total | 46.207 | 28 | |||
| Weight | 0.289 | 0.751 | |||
| Between groups | 114.873 | 2 | 57.436 | ||
| Within groups | 5159.817 | 26 | 198.454 | ||
| Total | 5274.690 | 28 | |||
| Height | 0.602 | 0.555 | |||
| Between groups | 526.909 | 2 | 263.454 | ||
| Within groups | 11369.850 | 26 | 437.302 | ||
| Total | 11896.759 | 28 | |||
| Multiple Comparisons; Tukey HSD | |||||||
|---|---|---|---|---|---|---|---|
| Dependent Variable | (I) Classification | (J) Classification | Mean Difference (I-J) | Std. Error | P-Value | 95% Confidence Interval | |
| Lower Bound | Upper Bound | ||||||
| Rate of Hospitalization | Benign | VUS | 1.267 | 0.884 | 0.339 | -0.93 | 3.46 |
| Pathogen | 1.183 a | 0.455 | 0.039 | 0.05 | 2.31 | ||
| VUS | Benign | -1.267 | 0.884 | 0.339 | -3.46 | 0.93 | |
| Pathogen | -0.083 | 0.897 | 0.995 | -2.31 | 2.15 | ||
| Pathogen | Benign | -1.183 a | 0.455 | 0.039 | -2.31 | -0.05 | |
| VUS | 0.083 | 0.897 | 0.995 | -2.15 | 2.31 | ||
Abbreviation: VUS, a variant of uncertain significance.
a The mean difference is significant at the 0.05 level.
5. Discussion
This study aimed to determine the genetic and clinical characteristics of children with FMF in northeastern Iran. A descriptive-analytical study was conducted, investigating 29 patients with FMF. This disease typically manifests at a young age, with 90% of patients experiencing their first attack before the age of 20. Although the onset of the disease can occur at an older age, it becomes rare after the age of 40 (13). The date of FMF onset is usually established through patient history; however, misinterpretation and misdiagnosis can sometimes occur (14). In this study, the average age of the first attack was 3.29 ± 2.54 years, with the oldest onset being at 13 years of age. These findings align with Tamir et al.'s (15) study, which reported that only 0.5% of patients experienced their first attack after reaching the age of 40. In contrast, Sayarlioglu et al. (16) reported that 5 patients (8.8%) had their first attack after the age of 40. This difference may be attributed to variations in the study population, as our study exclusively included children.
While most studies have reported that FMF affects both sexes equally (13), in our patient cohort, the ratio of women to men was approximately 1.4, with the majority of patients (58.3%) being female. Similarly, Ureten et al.'s (17) study found that the majority of patients were female (female/male: 1.85). However, in a Turkish study (16), 80% of FMF patients were male. Another report from northwest Iran indicated a higher prevalence of this disease in males (1: 7: 1) (18). Familial Mediterranean fever is a disease characterized by recurrent acute fever episodes, along with the involvement of various body parts, including the abdomen, chest, joints, muscles, scrotum, and skin. Abdominal pain is present in approximately 95% of patients, and the clinical presentation often resembles acute peritonitis (19). In our study, we observed that abdominal pain (51.7%) and fever (100%) were the most common clinical findings in our patients, consistent with findings in most other studies (19, 20). However, Tsuchiya-Suzuki et al. (21) reported that only 55% of their patients experienced abdominal symptoms. Our study also revealed that 44.8% of FMF cases exhibited arthritis, and 6.9% had chest pain, findings in line with other studies (19, 20). These symptoms had a lower prevalence compared to fever and abdominal pain. Among patients with arthritis, the knee joint (37.9%) was the most commonly affected site. Notably, myalgia was not observed in any of the patients. Tunca et al. (19) similarly concluded that arthritis, myalgia, and erythema erysipelas were significantly less common among adult-onset patients.
All patients who continued colchicine treatment exhibited a satisfactory response, with 75.7% achieving a complete response and 21.3% showing a partial response. These findings are consistent with other studies (15, 16), suggesting that colchicine is highly effective in treating FMF, and lower colchicine doses (1 mg/day) may be sufficient. Test results revealed elevated levels of acute phase reactants during FMF attacks, with all patients in our study experiencing an increase in the average number of white blood cells, ESR, and CRP. These results align with other reports (16, 22), which have demonstrated that ESR, CRP levels, and leukocyte counts significantly exceed the normal range during FMF attacks.
Concerning the genetic aspect of FMF, our study identified 23 patients as heterozygous, while 6 were homozygous for MEFV gene mutations. These results were consistent with Dundar et al.'s study (23), in which the majority of mutations were found to be heterozygous. In our study, the most prevalent heterozygous mutation was associated with the E148Q gene, with a frequency of 31%. However, in Salehzadeh et al.'s study (18), the most common heterozygous mutation was V726A-M694V, with a frequency of 10.46%. The predominant genotype in our study was E148Q, accounting for 41.3% of cases. This diverges from other studies where the most frequent genotypic mutation was M694V (12).
Among patients of Turkish and Armenian descent, M680I is typically the second most common mutation after M680V (24). Nevertheless, in our study, M680I emerged as the second most common mutation following E148Q, with a frequency of 10.2%, while the M694V mutation ranked third at 6.8%. In Jewish studies, M694V, V726A, and E148Q were more common than M680I (25). In studies involving Jewish and Arab populations (26, 27), M680I was reported as a rare mutation, whereas V726A was found to be more common in Armenians than in other populations (24). Among our patients, the 3 most prevalent mutations were M694V, M680I, and E148Q, which also happen to be the most common mutations reported in Mediterranean populations but were absent in Japanese populations. Most Japanese patients carried M694I on at least one allele, whereas this mutation was present in only 1 of our patients (21). Furthermore, we identified rare mutations, such as R761H and R408Q, with a higher detection rate compared to other populations. This observation may be attributed to consanguineous marriages and a high prevalence of siblings among our patients.
Our study does have some limitations. One limitation is the lack of reliable and complete family history to construct the genealogy of patients, including factors such as parental separation or the death of a family member, which could hinder accurate genetic analysis. Additionally, a comprehensive examination of all genotypes, including heterozygous and compound heterozygous mutations, would provide a more accurate frequency of genotypes among affected patients.
Considering the prevalence of FMF in the western regions of Iran and the Mediterranean regions, and given the increasing number of studies conducted in these areas, our study results indicate a lower prevalence of this disease in the eastern part of Iran. Based on the data obtained from this study, we suggest that other regions, regardless of the prevalence of this disease in Mediterranean regions, should also conduct research in this field. Further investigations will yield more comprehensive and precise results regarding the genetic and clinical profile of this disease throughout the country. Thus, we have demonstrated a lower prevalence of this disease in the eastern part of the country as well, contrary to expectations. Indeed, additional investigations are warranted to provide a more comprehensive and precise understanding of the genetic and clinical aspects of this disease nationwide. Therefore, according to our sample, there is a possibility of discovering more mutations in future studies, and other regions of Iran can gather comprehensive information about the state of the disease in the country through a registry study.
5.1. Conclusions
Our findings reveal that the most common genotype in our study was E148Q, which is considered benign. M680I and M694V were the most frequent pathogenic mutations, each occurring in 5 cases, indicating that FMF disease is rare in Northeastern Iran, and the number of pathogenic mutations is lower compared to Northwest Iran and other studies. Further investigations with a larger patient cohort and a comprehensive examination of the MEFV gene are necessary to establish the relationship between FMF symptoms and genotypes, which can aid in the treatment and management of the disease and its potential complications, such as amyloidosis.