1. Background
The emerging infectious disease COVID-19 has raised global concerns due to its potential to threaten global health. This has led to extensive investigation into unique clinical diseases and immunological changes connected to this infection (1, 2). The disease has been designated COVID-19 by the WHO in February 2020. The World Health Organization (WHO) declared COVID-19 a pandemic in 2021. As of December 2022, this pandemic had caused 633.0 million confirmed cases and 6.0 million deaths (WHO, 2022). COVID-19 can cause asymptomatic cases to severe manifestations with acute respiratory failure, septic shock, and multiple organ damage, which can be fatal (3-5). All age groups, including children, are experiencing more multi-organ dysfunctions, including cardiac issues (6, 7). Myocarditis, heart failure, arrhythmias, and other problems can occur in children with myocardial damage (8). COVID-19 infection causes heart impairment; however, the mechanisms are unknown. Several researchers believe that hypoxic and ischemic damage from coronary microvessel impairment, coronary artery disease, acute myocarditis, or systemic inflammatory reaction causes adult myocardial damage (6). Acute infection may cause acute cardiac dysfunction, post-viral immune response, and systemic hyperinflammation. In predisposed people, this cascade causes myocardial inflammation and dysfunction (7). Endothelitis, which involves endotheliocyte dysplasia and activation, is the main morphological expression of COVID-19 myocardial injury, according to several studies. This pathogenic process causes hemorrhages, intramural artery thrombosis, and necrosis (6, 8). Due to ACE2 production, COVID-19 infection causes substantial organ damage. ACE2, which is highly expressed in cell-free phagocytosis and macrophages, helps viruses spread from the lungs to other organs via the blood (8, 9). Several potential causes of myocardial damage have been identified, including elevated levels of cytokines and an immune-inflammatory response. Oxidative stress and cardiac cell injury can result from severe acute respiratory syndrome due to corona virus 2 (SARS-CoV-22) directly entering cardiomyocytes, pulmonary insufficiency, and hypoxia. The pathophysiological mechanism of myocardial injury in SARS-CoV-2 infection is poorly understood, despite the growing literature on cardiac involvement in children (10).
Due to the lower number of pediatric COVID-19 cases compared to adults, cardiovascular involvement in children is still poorly understood. Up to 34% of Spanish Pediatric Intensive Care Unit (PICU)
infants with COVID had cardiac dysfunction (11). As with respiratory syncytial virus or influenza, children with cardiac abnormalities may develop cardiac complications or a more severe SARS-CoV-2 infection (12). From April 2020, more healthy children in Europe and America have developed hyper-inflammatory and Kawasaki-Shock syndrome signs (13).
The aim of this study is to provide a comprehensive description of the cardiac manifestations observed in children who have been admitted to the pediatric intensive care unit as a result of SARS-CoV-2 infection.
2. Methods
2.1. Study Design
This is a cross-sectional prospective observational study that evaluated data from 178 patients from April 2022 to April 2023. We tracked 105 patients hospitalized in the PICU COVID-19 isolation unit at Minia University Hospital. Out of this total, 80 patients had a confirmed COVID-19 infection. The cross-sectional study examined the cardiac effects of COVID-19 on children hospitalized at Minia University Hospital's Pediatric Department's PICU Isolation Unit (Figure 1).
The study was conducted in Minia City, Egypt (with a population of 9.057 million as of December 31, 2022). Minia University Hospital's PICU was the sole tertiary COVID-19 service for children in this city. The pandemic did not affect PICU admission standards for regular cases, but we established COVID-19 isolation units. Patient numbers at Minia University Hospital can forecast pediatric COVID-19 patients with cardiac symptoms.
Eighty patients were divided into two groups: Group I comprised 46 COVID-19-positive cardiac patients, while group II consisted of 34 COVID-19 individuals without cardiac problems. This study included infants and children under 18 years old with serious illnesses who were hospitalized and had positive COVID-19 test results. We excluded patients aged less than one month or over 18 years who had no laboratory confirmation of COVID-19 infection, who could not complete the required laboratory and radiological investigations, and those whose families refused to participate.
2.2. COVID-19 Diagnostic Critera
A positive real-time PCR test for SARS-CoV-2 or positive blood samples for COVID antibodies (IgG and/or IgM) indicate COVID-19 infection (1).
2.3. Criteria for Diagnosis of Cardiac Complications
Diagnosis of cardiac complications is determined by clinical examination, which includes assessing for tachycardia and symptoms of heart failure. Laboratory investigations, such as elevated cardiac enzymes like troponin I, along with echocardiography (ECHO) findings (e.g., EF < 50%, FS < 25%), and electrocardiography (ECG) findings, such as arrhythmias or bradyarrhythmias (e.g., first-degree atrioventricular (AV) block, second-degree AV block, complete AV block, sinus bradycardia, and bundle branch block) are also considered (14).
2.4. Data Collection
All patients underwent a comprehensive history-taking and clinical assessment, which included general and cardiac examinations. Laboratory tests included complete blood count (CBC), C-reactive protein (CRP), D-dimer, serum ferritin, and troponin. Radiological tests comprised chest CT, cardiac evaluation, ECHO, and ECG.
2.5. Laboratory Investigation
To diagnose COVID-19, IgM and IgG antibody levels were measured using a chemiluminescence immunoassay. COVID-19 was detected using the DT Light 4 Real-Time PCR System (DNA Technology, Russia). The software DT Lite 4 7.9 interprets the results. The quantitative turbidimetric ferritin-turbinates test measured inflammatory markers.
2.6. At the Time of the COVID-19 Diagnosis
All ECGs were evaluated from the patients' medical records for cardiac evaluation. We used 12-lead ECG recordings with the patient lying down at 25 mm/s and 10 mm/mV. For ECHO, we utilized a GE Medical System, Horten, Norway, Vivid T8X ultrasound machine with a G.563.5 MHz multi-frequency transducer. Systolic and diastolic right ventricular diameters (RVD) and left ventricular end-systolic dimensions (LVESD) were assessed. Left ventricular ejection fraction (LV E F%), calculated as (LVEDD)3 - (LVESD)3/(LVEDD)3 × 100%, measures left ventricular systolic function. Additionally, LV fractional shortening (FS%) was determined as (LVEDD)(LVESD)/(LVEDD) × 100%. The E/A ratio measured passive LV filling and atrial contraction to assess left ventricular diastolic function.
2.7. Statistical Analysis
Statistical analysis was conducted using SPSS 26.0. The Mann-Whitney test was employed to compare non-parametric quantitative data between the two groups, while the chi-square test was used to compare qualitative data between the groups. The Pearson correlation coefficient test was utilized to assess the positive or negative association between two variables. Results were considered significant if P ≤ 0.05 and highly significant if P < 0.01.
2.8. Ethical Considerations
The Minia University Faculty of Medicine Ethics Committee approved this study, and it adhered to all necessary requirements. Written consent was obtained from each parent, ensuring participant anonymity and confidentiality. Deception was avoided, and participants were given the option to withdraw from the research. Approval No. 315:4/2022 was obtained.
3. Results
In a prospective cross-sectional study, we investigated the cardiac effects of COVID-19 on children. Eighty children were divided into two groups for this investigation. Group I comprised 46 COVID-19-positive individuals with cardiac complications, while group II included 34 individuals without cardiac abnormalities. Our study revealed that patients without cardiac abnormalities had a median age of seven years with an interquartile range (IQR) of 4 – 10.3, while those with cardiac complications had a median age of eight years. Among patients with cardiac problems, there were 13 males (28.2%) and 33 females (71.7%). No major demographic differences were observed between the groups (Table 1).
| Variables | Cardiac Complications | P-Value e | |
|---|---|---|---|
| Yes (N = 34) | No (N = 46) | ||
| Age (y); median (IQR) | 7 (4 - 10.3) | 8 (4 - 10.3) | 0.689 |
| Sex | 0.693 | ||
| Male | 11 (32.4) | 13 (28.3) | |
| Female | 23 (67.6) | 33 (71.7) | |
| BMI; mean ± SD (range) | 19.1 ± 2.2 (16 - 22.5) | 19.1 ± 2(16.3 - 22.9) | 0.980 |
| Residence | 0.154 | ||
| Urban | 21 (61.8) | 21 (45.7) | |
| Rural | 13 (38.2) | 25 (54.3) | |
| History of exposure | 0.837 | ||
| +Ve | 20 (58.8) | 20 (43.5) | |
| -Ve | 14 (41.2) | 26 (56.5) | |
Abbreviation: BMI, body mass index.
a Values are expressed as No. (%) or Mean ± SD.
b Independent Samples t-test for parametric quantitative data between the two groups.
c Mann Whitney test for non-parametric quantitative data between the two groups.
d Chi-square test for qualitative data between the two groups.
e Significant level at P-value < 0.05.
All of our patients underwent ECGs, with 58.8% showing normal results (47 children) and 41.3% showing arrhythmias (33 children). These abnormal ECG findings included: 9% of patients (3 children) with supraventricular tachycardia (SVT), 7.5% with bundle branch block (BBB), 3.8% with first-degree AV block, 5% with a long PR interval, 5% with a long QT interval, 15% (12 individuals) with abnormal ST or T wave segments, and 1.3% with a pathological Q wave (Table 2).
| ECG | Descriptive Statistics (N = 80) |
|---|---|
| Normal | 47 (58.8) |
| Arrhythmias | 33 (41.3) |
| Tachyarrhythmia (SVT) | 3 (3.8) |
| BBB | 6 (7.5) |
| First-degree AV block | 3 (3.8) |
| Prolonged PR interval | 4 (5) |
| Prolonged QT interval | 4 (5) |
| Abnormal ST or T wave segment | 12 (15) |
| Pathological Q | 1 (1.3) |
Abbreviations: ECG, electrocardiogram; BBB, bundle branch block; AV block, atrioventricular block; SVT, supraventricular tachycardia.
a Values are expressed as No. (%).
Forty two and a half percent had no cardiac problems, while 57.5% experienced issues. Among them, 41.3% had arrhythmias, 31.3% had myocardial dysfunction, 7.5% had bundle branch block (BBB), 1.3% had pancarditis, and 3.8% had first-degree AV block (Table 3).
| Variables | Descriptive Statistics (N = 80) | |
|---|---|---|
| Yes | No | |
| Cardiac complication | 46 (57.5) | 34 (42.5) |
| Tachyarrhythmia | 3 (3.8) | 77 (96.25) |
| Myocardial dysfunction | 25 (31.3) | 55 (68.8) |
| BBB | 6 (7.5) | 74 (92.5) |
| Pan carditis | 1 (1.3) | 79 (98.8) |
| Heart block | 5 (6.3) | 75 (93.8) |
Abbreviations: BBB, bundle branch block.
a Values are expressed as No. (%).
The only difference between groups was death, which was significantly higher in cardiac patients (P = 0.004). The median hospitalization days for cardiac patients were 15 (10.5 – 18), while those without cardiac concerns were 14.5 (10.5 – 18), with a P value of 0.252. Our study reported 17 (37%) cardiac complications and 3 (8.8%) non-cardiac deaths, P = 0.004 (Table 4 and Figure 2).
| Variables | Cardiac Complications | P-Value | |
|---|---|---|---|
| Yes (N = 34) | No (N = 46) | ||
| Hospitalization days; median (IQR) | 14.5 (10.5 - 18) | 15 (11.8 - 19.5) | 0.252 |
| Mortality | 0.004 d | ||
| Survival | 31 (91.2) | 29 (63) | |
| Non survival | 3 (8.8) | 17 (37) | |
a Values are expressed as No. (%).
b Mann Whitney test for non-parametric quantitative data between the two groups.
c Chi-square test for qualitative data between the two groups.
d Significant level at P-value < 0.05.
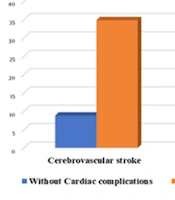
Creatinine (OR = 9.583, P = 0.027) was the best predictor of cardiac problems in logistic regression. Troponin positivity, sepsis, stroke, respiratory distress, neutrophils, and platelets followed suit. Refer to (Table 5 and Figure 3).
| Variables | OR | 95% CI | P-Value b |
|---|---|---|---|
| Respiratory distress | 1.181 | 1006 - 1.385 | 0.042 |
| Sepsis | 6.057 | 1.606 - 22.851 | 0.008 |
| Cerebrovascular stroke | 5.511 | 1.456 - 20.866 | 0.012 |
| Lymphocytes | 0.94 | 0.898 - 0.985 | 0.009 |
| Neutrophils | 1.04 | 1.003 - 1.007 | 0.033 |
| Platelets | 1.007 | 1.001 - 1.013 | 0.017 |
| Cr | 9.583 | 1.289 - 71.259 | 0.027 |
| Troponin (+Ve) | 6.786 | 2.438 - 18.886 | < 0.001 |
| FS | 0.798 | 0.713 - 0.894 | < 0.001 |
| EF | 0.845 | 0.776 - 0.92 | < 0.001 |
Abbreviations: Cr, creatinine; FS, fraction shortening; EG, ejection fraction; OR, odds ratio; CI, confidence interval.
a Logistic regression analysis.
b Significant level at P-value < 0.05.
4. Discussion
Wuhan, China, experienced a new outbreak of coronavirus-related pneumonia in December 2019. Due to its rapid spread, the WHO declared COVID-19 a pandemic on March 11, 2020. Symptoms of COVID-19 are identical in children and adults (15). This study aimed to investigate COVID-19-related cardiac manifestations in children. Our research revealed a mean age of 7 years with a range of 4 – 10 years, consistent with Blumfield and Levin (16), who observed COVID-19 infection in children at a mean age of 7 years. The patients comprised 70% females and 30% males, aligning with the findings of Götzinger et al. (17). There were no significant demographic differences between the two groups. These findings suggest that demographic characteristics may not predict cardiac issues in these patients, consistent with Xu et al. (18). We also found that 57.5% of COVID-19-infected children suffered from cardiac conditions, supporting the findings of Valverde et al. (19). Unlike Song and Kwon (20), few children with acute COVID-19 infection had cardiac complications. The presentation and severity of COVID-19 were examined. Our investigation revealed 31.3% myocardial dysfunction, similar to the findings of Valverde et al. (19). Other cardiac complications in our study included 41.3% arrhythmias, 7.5% BBB, 3.8% first-degree AV block, and 1.3% pancarditis. Arrhythmia can result from lung viral hypoxia, myocarditis, an abnormal host immunological response, myocardial ischemia, pulmonary hypertension-induced cardiac strain, electrolyte disturbance, intravascular volume imbalances, and pharmacological side effects (20). All our patients underwent ECG testing, and 41.3% showed abnormalities, consistent with the findings of Valverde et al. (19). Sepsis and cerebrovascular stroke were significantly associated with cardiac complications in our study, suggesting possible links between these diseases and cardiac involvement. Appavu et al. (21) found that 2% – 6% of SARS-CoV-2 patients had cerebrovascular involvement. Similar to Heubner et al. (22), our investigation found elevated ESR, CRP, LDH, and troponin I levels in cardiac patients. Neutrophils, platelets, and creatinine were higher in COVID-19 patients, especially those with cardiac complications. According to Mittal et al. (23), reduced cardiac output to both kidneys increases the risk of renal dysfunction in SARS-CoV-2-infected children. Cardiovascular patients had reduced lymphocyte percentages. According to Wang et al. (24), 70.3% of patients had lymphopenia. Further research is needed to understand the clinical effects and mechanisms of these associations. Patients with cardiac problems were compared to those without regarding troponin I levels. Troponin I levels were considerably higher in patients with cardiac problems. Shi et al. (25), who showed higher troponin I levels in patients with cardiac injury than those without, suggest that this patient population may have a higher risk of cardiac problems. Patients with and without cardiac problems were compared based on CT chest grading, but no significant differences in CT grade were identified across groups. In this patient sample, CT lung involvement severity did not predict cardiac issues. Oxygen therapy, mechanical ventilation, antiviral medicine, and corticosteroids did not differ between cardiac and non-cardiac patients. This contrasts with Gamberini et al. (26), who found that cardiac patients required more mechanical ventilation and a longer duration of treatment. In our research trial, cardiac and non-cardiac patients had similar hospitalization days. These findings suggest that cardiac issues may not significantly prolong hospitalization in this patient population. However, Zeng et al. (27) found that fulminant myocarditis exacerbated COVID-19 infection and increased hospitalization and ICU stays. COVID-19 individuals with cardiac issues exhibited a higher death rate in our study. This supports Sinha et al. (28) in suggesting that cardiac problems increase mortality. Our investigation compared ECG and echocardiography data between patients with cardiac issues and those without, as Rodriguez-Gonzalez et al. did (29). Fifty-seven percent of instances showed myocardial dysfunction, and 27% displayed ECG abnormalities, including arrhythmias. However, Ersoy Dursun et al. (30) discovered EF < 55% in one patient, while the others had normal values. Logistic regression predicted COVID-19-related cardiac issues. Sepsis, stroke, respiratory distress, and increased creatinine levels predicted cardiac involvement. A higher fractional shortening (FS), ejection fraction (EF), and lymphocyte count were protective factors. More research is needed on the implications of these variables for COVID-19 cardiac patients. These mechanisms were supported by increased neutrophil, platelet, ESR, and troponin I levels in ECG-defined arrhythmia patients (20). The mechanisms include direct viral lung hypoxia, myocarditis, cardiac ischemia, and aberrant host immunological responses. CT grading was the same for COVID-19 patients with and without arrhythmias. Arrhythmias were positively linked with platelets, ESR, and troponin I positivity in COVID-19 patients, but all other correlations were inconsequential. Platelet activation may cause COVID-19-induced arrhythmias due to their close connection. COVID-19 inflammation and endothelial damage involve platelet activation and aggregation (31). The high correlation between ESR and arrhythmias implies systemic inflammation may cause heart issues. Suding et al. (32) found that viral infection and systemic illness with an abnormal host immune response can cause arrhythmias. The strong connection between troponin I positive and arrhythmias suggests that COVID-19 patients with arrhythmias may have higher cardiac injury. Due to myocardial damage, COVID-19 patients with high troponin I had worse outcomes. High troponin levels were likely generated by heart muscle stress from inflammation, as ECHO and ECG showed no coronary insufficiency or ST-segment changes, according to Santi et al. (33). This patient may have had cardiac arrhythmia from inflammatory stress and metabolic alterations (33). Some limitations exist in this study. First, this study's sample of hospitalized pediatric COVID-19 patients is small. Thus, data from a broader cohort might help comprehend this population. Our study lacked cardiovascular magnetic resonance imaging data to confirm myocardial edema, necrosis, and/or microvascular dysfunction. However, infection control limits hampered acute cardiac MRI.
4.1. Conclusions
More data are needed on COVID-19's cardiovascular effects on children. We found that children hospitalized with acute COVID-19 should undergo cardiac exams and cardiovascular observation. This helps detect and promptly treat life-threatening cardiac conditions. Cardiovascular involvement predicts the outcome of COVID-19. Multidisciplinary teams managing COVID-19 in children must include cardiology expertise. To reduce morbidity and mortality from cardiovascular sequelae, resources must be available to effectively identify and treat cardiac conditions. A comprehensive evaluation including ECG, echocardiogram, and basic cardiac examination is necessary to promptly identify and address any issues.