1. Background
Hypermobility spectrum disorder (HSD), likely a multifactorial condition characterized by musculoskeletal pain, joint instability, and reduced bone mineral density (BMD) (1), may also present with fatigue and other systemic symptoms affecting daily functioning (2). The genetic basis of hypermobility syndrome can be autosomal dominant or recessive, potentially affecting collagen types one, three, and five (3, 4). Hypermobility spectrum disorder manifests in varying degrees, with the most severe being Ehlers-Danlos disease, although it is noteworthy that symptoms of hypermobility syndrome typically emerge in a minority of cases, with many individuals experiencing pain symptoms in old age (5, 6).
Hypermobility spectrum disorders are characterized by joint hypermobility assessed using the Beighton score, coupled with one or more secondary musculoskeletal manifestations such as post-traumatic symptoms, pain, altered proprioception, and various features including pes planus, kyphosis, scoliosis, or joint misalignment (2, 7).
Benign joint hypermobility syndrome (BJHS) represents a type of connective tissue disorder marked by hypermobility, presenting with widespread musculoskeletal symptoms devoid of rheumatological findings (8). Symptomatic hypermobility of multiple joints (9) is typically the initial sign, often leading to poor exercise tolerance, arthralgia, and recurrent subluxations (8, 10). This disorder is frequently overlooked due to vague symptoms and mild clinical severity, resulting in delayed diagnosis or misidentification with other conditions (11). Low bone density, characterized by bone density lower than expected for age and gender (12), may be linked to collagen defects in hypermobility disorders, potentially impacting BMD (13). Osteoporosis, a metabolic bone disorder characterized by decreased bone mass and strength, heightens the risk of fractures (14). In children, osteoporosis is defined by one vertebral fracture or a Z-score below -2, along with at least one lower extremity long bone fracture, or two upper extremity long bone fractures based on age (15). Limited research on joint hypermobility disorders suggests a propensity towards osteopenia, albeit with conflicting findings (16).
2. Objectives
Differentiating between these two conditions and understanding their distinct effects on the musculoskeletal system and bone density can aid in more accurate diagnosis and the implementation of tailored treatments. Hence, recognizing the significance of this topic, we aimed to evaluate and compare bone density in children with HSD and those with benign hypermobility.
3. Methods
This case-control survey was conducted on 73 children with HSD and children with generalized hypermobility referred to the Rheumatology clinic of Mofid Hospital in Tehran from February to November 2022. Sampling was performed using a convenience method. The inclusion criteria for patients were a documented Beighton score ≥ 6 in prepubertal children and adolescents (aged 3 - 16 years). Exclusion criteria included known rheumatic disorders, other forms of connective tissue diseases, contraindications to a DXA scan, spinal cord disorders, and parental dissatisfaction with their child's participation in the Study. Beighton's criteria for assessing hypermobility were recorded on a 0 - 9 point scale based on passive dorsiflexion of the fifth finger ≥ 90°, passive apposition of the thumb to the forearm, elbow hyperextension ≥ 10°, knee hyperextension ≥ 10°, and the ability to rest the palms on the floor while bending forward with straight knees (17). The Beighton score was determined through the clinical examination, with a total score of ≥ 6 considered as the threshold for diagnosing general joint hypermobility. Eligible patients were divided into two groups: The case group comprised patients referred with complaints of non-inflammatory musculoskeletal pain who met the necessary Beighton criteria (HSD group: n = 24), while the control group consisted of patients who presented to the rheumatology clinic for any reason and demonstrated benign hypermobility during examination (n = 49). Patient body weight was measured using a calibrated SECA balance measuring scale, and their height was recorded. Bone mineral density was assessed using dual-energy x-ray absorptiometry (DEXA) by determining the z score from L1-L4 and whole body less head. This study was approved by the ethics committee of Shahid Beheshti University of Medical Science (IR.SBMU.MSP.REC.1400.023), and written informed consent was obtained from all participants, their parents, or legal guardians prior to study enrollment.
3.1. Statistical Analysis
Data analysis was performed using SPSS version 26 statistical software. Quantitative and qualitative variables were presented as mean ± SD and number (percentage), respectively. Kolmogorov–Smirnov and Shapiro–Wilk tests were utilized to assess distribution. The comparison of bone density between the two groups was conducted using an independent t-test. The chi-square test was employed to compare qualitative data between the groups. Linear regression and logistic modeling were utilized to control for confounding variables. A significance level of P < 0.05 was adopted.
4. Results
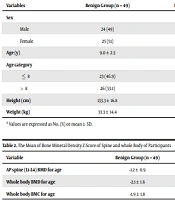
In this case-control study, 73 patients with hypermobility were included, with 49 patients (67.1%) in the benign hypermobility group and 24 patients (32.9%) in the HSD group. Among the patients, 49.3% were male. The mean age of the participants was 9.1 ± 2.8 years. In the Benign Hypermobility and HSD groups, 49% and 50% were boys, respectively. There was no significant difference in gender distribution between the two groups (P = 0.93). The mean height of the participants was 134.7 ± 17.5 centimeters, and there was no significant difference in height between the two groups (P = 0.33). The mean weight in the benign group and HSD group was 33.3 ± 14.4 and 38.4 ± 16.2 kilograms, respectively, with no difference observed between the two groups (P = 0.17). The demographic features are shown in Table 1.
| Variables | Benign Group (n = 49) | HSD Group (n = 24) | Total (n = 73) | P-Value |
|---|---|---|---|---|
| Sex | 0.93 | |||
| Male | 24 (49) | 12 (50) | 36 (49.3) | |
| Female | 25 (51) | 12 (50) | 37 (50.7) | |
| Age (y) | 9.0 ± 2.5 | 9.4 ± 3.4 | 9.1 ± 2.8 | 0.56 |
| Age category | 0.80 | |||
| ≤ 8 | 23 (46.9) | 12 (50) | 35 (47.9) | |
| > 8 | 26 (53.1) | 12 (50) | 38 (52.1) | |
| Height (cm) | 133.3 ± 16.8 | 137.6 ± 19.0 | 134.7 ± 17.5 | 0.33 |
| Weight (kg) | 33.3 ± 14.4 | 38.4 ± 16.2 | 35.0 ± 15.1 | 0.17 |
a Values are expressed as No. (%) or mean ± SD.
The mean BMD z-scores of the spine and the whole-body for all patients were -0.8 ± 1.2 and -1.8 ± 1.5, respectively. The AP spine (L1-L4) BMD showed a significant difference between the benign and HSD groups (P = 0.001). Additionally, the whole-body BMD differed significantly between the two groups (P = 0.008), while there was no considerable difference in whole-body BMC between the groups (P = 0.06). Further details are provided in Table 2.
| Variables | Benign Group (n = 49) | HSD Group (n = 24) | Total (n = 73) | P-Value |
|---|---|---|---|---|
| AP spine (L1-L4) BMD for age | -1.2 ± 0.9 | 0.4 ± 1.3 | -0.8 ± 1.2 | 0.001 |
| Whole body BMD for age | -2.1 ± 1.6 | -0.9 ± 1.1 | -1.8 ± 1.5 | 0.008 |
| Whole body BMC for age | -1.9 ± 1.8 | -0.9 ± 1.3 | -1.6 ± 1.7 | 0.06 |
Abbreviations: BMD, bone mineral density; BMC, bone mineral content.
Based on the BMD Z-score of the spine, in the HSD group, 18 patients (81.8%) had normal BMD, while 4 patients (18.2%) had low bone density; osteoporosis was not observed in the HSD group patients. In the Benign Hypermobility group, according to the BMD Z-score of the spine, 36.5% had a normal condition, 56.2% exhibited low bone density, and 7.3% showed signs of osteoporosis.
The two groups showed a significant difference in terms of the frequency distribution of the spine based on the BMD Z-score (P = 0.002). Furthermore, a notable statistical difference was observed in the frequency distribution of the whole-body bone condition based on the BMD Z-score (P = 0.04) (Table 3).
| Group | Normal BMD | Low Bone Mass | Osteoporosis | P-Value |
|---|---|---|---|---|
| Frequency distribution of spine bone status | 0.002 | |||
| Benign group (n = 41) | 15 (36.5) | 23 (56.2) | 3 (7.3) | |
| HSD group (n = 22) | 18 (81.8) | 4 (18.2) | 0 (0) | |
| Total (n = 63) | 33 (52) | 27 (43) | 3 (5) | |
| Frequency distribution of whole body bone status | 0.04 | |||
| Benign group (n = 38) | 9 (32.7) | 14 (36.8) | 15 (39.5) | |
| HSD group (n = 15) | 9 (60) | 3 (20) | 3 (20) | |
| Total (n = 53) | 18 (34) | 17 (32) | 18 (34) |
Abbreviation: HSD, hypermobility spectrum disorder.
a Values are expressed as No. (%).
According to our findings, the results of Pearson's correlation test showed that there is no significant relationship between the Z-score of the spine and the whole body with age and gender (P > 0.05). However, a significant relationship was observed between the Z-score of the spine and the Z-score of the whole body (P < 0.001 and r = 0.64).
5. Discussion
In this study, we assessed bone mineral density in HSD and benign hypermobility patients. Our findings showed that AP spine (L1-L4) BMD and whole body BMD were statistically different between the benign group and HSD groups, while there was no considerable difference in whole-body BMC between the two groups (P = 0.06). Moreover, based on the BMD Z-score of the whole body and the spine, osteoporosis in the benign group was higher than in the HSD group, and a statistically significant difference was observed (P < 0.05).
In the study by Gulbahar et al., which aimed to investigate bone density in pre-menopausal women with joint hypermobility, the results showed a decrease in bone mineral density in patients with joint hypermobility compared to healthy subjects. Although there was a meaningful difference, particularly for the femur, in total femoral and trochanteric bone density, t and Z-scores, femoral neck, and Ward's triangle Z-score (16).
Mirsha et al. reported a decrease in bone density by determining the extra-articular features of benign hypermobility. They also noted that patients, especially those under 45 years of age, have reduced bone density compared to normal people of the same age. However, this was not statistically significant, and no relationship was observed between hypermobility grades and bone density. Conditions that may change bone mineral density were not investigated in their study (18). Nijs et al., in their cross-sectional investigation, noted that patients with benign hypermobility syndrome had lower t-scores for bone structure and bone strength data measured by ultrasound and tomography, but never reached threshold criteria. They did not reach for osteoporosis as suggested by WHO (19).
Engelbert et al. demonstrated that pediatrics with symptomatic generalized hypermobility had less bone density on bone ultrasound compared to controls or asymptomatic hypermobility, and that children with symptomatic hypermobility had more systemic impairment (20).
The study by Gulbahar et al. confirmed that there is a tendency to osteoporosis in benign hypermobility, and despite a lower tendency to osteoporosis compared to other hereditary connective tissue disorders, these patients can even reach the levels of osteoporosis. Osteoporosis in HCTD is caused by a combination of matrix structural abnormality and matrix failure. They noted that not only a quantitative decrease in bone density but also a qualitative change in bone collagen is likely to be the cause of bone fragility (16). Our findings were similar to the result of Gulbahar's study (16). In our study, osteoporosis in the benign group was significantly higher than in the HSD group. This finding may be attributed to HSD patients seeking more medical services compared to asymptomatic benign hypermobility patients who consume fewer medical services and complementary medications.
In the study by Ritelli et al. in Italy, it was determined by examining 75 patients with HSD that osteopenia (t score between -1 and -2.4) occurred in 35.5% and osteoporosis was present in 16.6% of adults with this disease, but in affected children, bone density was normal in all cases (21). In a comparative study by Dolan et al., comparing 23 cases of HSD with Ehlers-Danlos and 23 subjects in the control group, it was announced that the bone density measured by DEXA in the femoral neck and lumbar regions was significantly lower in the HSD group (22). In a review study, it was reported that in cases of HSD, the bone density decreases only a little, which is not accompanied by an increase in the risk of fractures, and it is usually seen at older ages, and there is a higher bone density at younger ages (23).
5.1. Conclusions
Considering the high rate of osteoporosis in benign patients, it is advisable to also consider the tendency to osteopenia in the follow-up of such patients. One limitation of this study is the small sample size, attributable to the study being conducted during the COVID-19 pandemic when patients did not seek medical attention for musculoskeletal pain. It is recommended that more multicenter studies with larger sample sizes be undertaken to measure bone mineral density and fracture risk in hypermobility patients, alongside assessing blood factors and vitamin D levels.