1. Background
Following great success in solid organ transplant over the past 50 years, excellent short-term survival of transplant tissue and its long-term adequate function without the development of significant associated comorbidities is typically expected (1). Immunosuppressive regimens have improved, meaning that acute graft rejection has been significantly reduced, and even chronic forms of graft rejection have been delayed, and their prevalence has decreased. As a result, the physicians' main attention is focused on the general health of recipients, and apart from allograft health in particular, cardiovascular health is an important component. In turn, each of the risk factors of cardiovascular disease (CVD), including dyslipidemia, has received part of the posttransplant management strategy in this population. Excess weight gain, hypertension, diabetes mellitus, and hyperlipidemia are common complications after liver transplant, most likely due to multiple causes. These elements of metabolic syndrome can raise the risk of CVD, a leading cause of death following liver donation. To reduce cardiovascular morbidity and mortality following liver transplantation, it is critical to comprehend the prevalence and predisposing factors of CVD in recipients, as well as to develop a thorough preventive and treatment plan. The cornerstones of every preventative and treatment plan are weight loss and metabolic syndrome control (2, 3).
According to estimates, hyperlipidemia affects between 27% and 71% of liver transplant patients. This is a significant prevalence rate (4-6). In our previous study, which spanned a period of 20 years and included 391 pediatric liver transplant recipients, the rates of posttransplant hyperlipidemia, hyperglycemia, and hypertension were found to be 7.5%, 22% and 9.6%, respectively; in addition, the rate of metabolic syndrome was 50.2%. In that study, the pretransplant rate of metabolic syndrome was 10.5% (7).
The development of hyperlipidemia is influenced by various factors in the context of liver transplants. Immunosuppressive drugs after liver transplantation may lead to abnormalities of lipid metabolism and hyperlipidemia (8).
According to a meta-analysis that gathered data from community-based and case-control studies, recipients of liver were found to have an approximately 64% higher risk of CVD compared to the general population. On the other hand, transplant recipients' higher risk of atherosclerosis-related illnesses suggests that lipid-lowering medications, pharmacological interactions, and other adverse effects are avoided for all of these patients. When it comes to administering lipid-lowering medications to liver transplant recipients, many transplant doctors are really hesitant (9).
For these individuals, the best care should involve ongoing monitoring and controlling cardiovascular risk factors, including dyslipidemia. Therefore, it is essential to consider recommendations for managing lipid levels in liver transplant recipients, including considering the risk factors of CVD and the effects of immunosuppressive agents (10).
While research on post-liver transplant dyslipidemia has focused on patient factors and immunosuppressive regimens, the role of liver donors in the development or prevention of this complication is not investigated properly.
Dyslipidemia in liver transplant recipients emerges as a complex interplay of factors prominently influenced by immunosuppressive medications, metabolic inflammation, and donor-related elements. The intricate nature of dyslipidemia prompts further exploration to discern whether it stems primarily from metabolic inflammation, dyslipidemia alone, or a synergistic combination of both. Elucidating the specific mechanisms driving dyslipidemia in this context is essential for devising targeted interventions. By unraveling the intricacies of these contributing factors, we can better tailor preventative and management strategies, ultimately reducing the risk of cardiovascular complications in this vulnerable population.
2. Objectives
The purpose of this study was to compare the lipid profiles and prevalence of dyslipidemia in children who underwent living-related (LR) or deceased donor (DD) liver transplants.
3. Methods
The present study is a retrospective cross-sectional study that was performed to evaluate and compare the status of lipid profiles in pediatric patients receiving liver transplants from LR and DD during 2005-2018. The study population consisted of all patients younger than 18 years who underwent transplant surgery in Shiraz (the main Pediatric Liver Transplant Center in Iran) and were followed up in clinics affiliated with Shiraz University of Medical Sciences.
Exclusion criteria were patients who died within 2 years of transplantation, patients with any pretransplant dyslipidemia (such as familial hypercholesterolemia), and patients who did not receive regular follow-up after transplantation. The patients were divided into 2 groups of LR donor and DD according to the source of the transplanted graft. Then, demographic information, indications of liver transplant, graft type, immunosuppressive drugs, and clinical and paraclinical findings were collected using their medical records. According to the American College of Sports Medicine, the normal triglyceride (TG) levels for children under 18 years of age are below 150 mg/dL. Borderline high values are considered to be in the range of 150 - 199 mg/dL, while high and very high values are categorized as 200 - 499 mg/dL and greater than 500 mg/dL, respectively. The American Academy of Pediatrics expresses that normal total cholesterol (TC) levels for children less than 18 years are less than 170 mg/dL, and borderline and high levels are 170 - 199 mg/dL and more than 200 mg/dL. Normal low-density lipoprotein (LDL) cholesterol levels are expressed at less than 110 mg/dL, and borderline and high levels are 110 - 129 mg/dL and more than 130 mg/dL. Fasting blood sugar (FBS) levels lower than 100 mg/dL were considered normal. Prediabetes was defined as FBS levels ranging from 100 - 125 mg/dL, while diabetes was defined as FBS levels equal to or greater than 126 mg/dL. In the present study, high-density lipoprotein (HDL) cholesterol levels above 50 mg/dL after 2 years of transplant were considered to be the optimal lipid profile in patients. Statistical analyses were performed to determine the predictive factors of this marker. Statistical analysis of fasting lipid and glucose profiles was performed on average with a 95% CI.
After collecting data and entering it into SPSS version 18, they were analyzed; then, descriptive indices, such as mean and SD, minimum and maximum, and frequency and percentage, were used. In inferential analysis to investigate the relationship between 2 categorized factors, the chi-square test was used. Independent t-test or its nonparametric equivalent, the Mann-Whitney U test, was used to compare the mean of a quantitative factor between the 2 groups. Also, a one-way analysis of variance (ANOVA) was used to compare the mean of a quantitative factor between the 3 groups. The Pearson correlation test was used to examine the correlation between the 2 quantitative factors. The level of significance at all tests was 0.05.
4. Results
This study was performed on 397 liver transplant recipients for 13 years. The mean age of recipients on the transplant day was 8.4 ± 4.7 years (range: 1 to 18 years). Among the patients, 182 (46%) were girls with a mean age of 8.4 ± 5.3 years, and 215 (54%) were boys with a mean age of 8.4 ± 2.2 years (P = 0.504).
Of the total population, 234 (58.9%) had received their transplant from DD and 161 (40.6%) from LR donors. The mean body mass index (BMI) in the LR and DD groups were 16.25 ± 3.29 and 17.51 ± 5.49, respectively (P = 0.05). The mean time elapsed after transplant in all 397 patients was 2.45 ± 5.74 years.
The most common underlying diseases were biliary atresia (22%) and autoimmune hepatitis (15%).
The frequency of any of the posttransplant complications in individuals was as follows: Hepatic artery thrombosis in 8 (2%), hepatic vein thrombosis in 17 (4.3%; one of them had portal vein thrombosis), graft rejection in 65 (16.4%), biliary complications in 16 (4%), infections in 17 (4.3%), ascites in 3 (0.8%), convulsion in 27 (6.8%), renal problems in 4 (1%), pulmonary heart problems in 4 (1%), posttransplant lymphoproliferative disorder (PTLD) in 3 (0.8%), and bowel perforation in 1 (0.3%). The rate of posttransplant complications was not related to sex and age groups (P > 0.05).
Table 1 presents the results of the independent t-test for comparing the mean values of lipid profile and FBS between girls and boys.
| Lipid Profile Factors and Gender | Number | Mean ± SD | Minimum | Maximum | P-Value |
|---|---|---|---|---|---|
| FBS | 0.37 | ||||
| Female | 179 | 86.88 ± 20.89 | 56.33 | 312 | |
| Male | 204 | 88.61 ± 17.22 | 63.33 | 203 | |
| TG | 0.06 | ||||
| Female | 179 | 13.47 ± 58.53 | 57.67 | 570 | |
| Male | 204 | 12.55 ± 45.57 | 46.67 | 289 | |
| TC | 0.37 | ||||
| Female | 179 | 16.45 ± 39.8 | 55 | 331.33 | |
| Male | 204 | 16.11 ± 51.97 | 59 | 570 | |
| HDL | 0.7 | ||||
| Female | 156 | 50.14 ± 21.89 | 6 | 259 | |
| Male | 185 | 49.08 ± 28.22 | 19 | 386 | |
| LDL | 0.78 | ||||
| Female | 156 | 87.5 ± 33.39 | 28 | 222.67 | |
| Male | 184 | 88.83 ± 50.46 | 11 | 595 |
Abbreviations: FBS, fasting blood sugar; TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
The results showed no significant differences between them in any of the relevant factors.
Table 2 shows the results of 1-way ANOVA for comparing the mean lipid profile and FBS between different age groups.
| Lipid Profile and Age Groups, y | Number | Mean ± SD | Minimum | Maximum | P-Value |
|---|---|---|---|---|---|
| FBS | 0.001 | ||||
| Under 6 | 150 | 84.13 ± 15.34 | 56.33 | 203 | |
| 7 - 12 | 140 | 87.88 ± 10.31 | 65.67 | 161 | |
| 13 - 18 | 92 | 93.74 ± 30.2 | 65.33 | 312 | |
| TG | 0.13 | ||||
| Under 6 | 150 | 123.95 ± 56.48 | 53.67 | 570 | |
| 7 - 12 | 140 | 124.6 ± 48.3 | 57.67 | 317.3 | |
| 13 - 18 | 92 | 136.88 ± 49.99 | 46.67 | 293.33 | |
| TC | 0.33 | ||||
| Under 6 | 150 | 166.7 ± 49.99 | 96.67 | 532.67 | |
| 7 - 12 | 140 | 159.09 ± 31.04 | 55 | 268 | |
| 13 - 18 | 92 | 159.86 ± 59.28 | 59 | 570 | |
| HDL | 0.04 | ||||
| Under 6 | 126 | 52.35 ± 22.68 | 21 | 259.5 | |
| 7 - 12 | 130 | 50.6 ± 32.64 | 6 | 286 | |
| 13 - 18 | 84 | 43.78 ± 12.63 | 19 | 129.5 | |
| LDL | 0.797 | ||||
| Under 6 | 126 | 87.24 ± 35.59 | 37 | 286 | |
| 7 - 12 | 129 | 87.75 ± 31.24 | 11 | 203 | |
| 13 - 18 | 84 | 91.14 ± 65.05 | 23.5 | 595 |
Abbreviations: FBS, fasting blood sugar; TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
There was a significant difference between mean FBS and HDL in different age groups (P < 0.05). In other words, with increasing age, the mean value of FBS increases, and the mean value of HDL decreases.
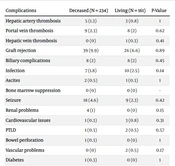
Table 3 presents the chi-square test to investigate the frequency distribution of post-liver transplant complications based on transplanted graft donors (LR vs DD).
| Complications | Deceased (N = 234) | Living (N = 161) | P-Value |
|---|---|---|---|
| Hepatic artery thrombosis | 5 (1.3) | 3 (0.8) | 1 |
| Portal vein thrombosis | 9 (2.3) | 8 (2) | 0.62 |
| Hepatic vein thrombosis | 0 (0) | 1 (0.3) | 0.41 |
| Graft rejection | 39 (9.9) | 26 (6.6) | 0.89 |
| Biliary complications | 8 (2) | 8 (2) | 0.45 |
| Infection | 7 (1.8) | 10 (2.5) | 0.14 |
| Ascites | 2 (0.5) | 1 (0.3) | 1 |
| Bone marrow suppression | 0 (0) | 0 (0) | - |
| Seizure | 18 (4.6) | 9 (2.3) | 0.42 |
| Renal problems | 4 (1) | 0 (0) | 0.15 |
| Cardiovascular issues | 1 (0.3) | 3 (0.8) | 0.31 |
| PTLD | 1 (0.3) | 2 (0.5) | 0.57 |
| Bowel perforation | 1 (0.3) | 0 (0) | 1 |
| Vascular problems | 0 (0) | 2 (0.5) | 0.17 |
| Diabetes | 1 (0.3) | 0 (0) | 1 |
Abbreviation: PTLD, posttransplant lymphoproliferative disorder.
There was no significant difference in the rate of posttransplant complications regarding the donor type (P < 0.05).
Hypertriglyceridemia and hypercholesterolemia were seen in 23.7% and 35.5% of the patients, respectively. Totally, 19.2% of the cases had abnormal LDL, and 65.6% had low HDL.
Comparing the mean lipid profile and FBS between the 2 donor groups (Table 4), the mean TG and HDL levels were significantly different between the 2 groups of DD and LR donors, with more favorable in the LR donor group (P < 0.05).
| Lipid Profile/Donor | Number | Mean ± SD | Minimum | Maximum | P-Value |
|---|---|---|---|---|---|
| FBS | 0.2 | ||||
| Brain death | 223 | 88.89 ± 20.63 | 56.33 | 312 | |
| Live | 158 | 86.31 ± 16.56 | 63.33 | 203 | |
| TG | 0.006 | ||||
| Brain death | 223 | 133.13 ± 49.51 | 46.67 | 336.33 | |
| Live | 158 | 118.19 ± 54.69 | 53.67 | 570 | |
| TC | 0.64 | ||||
| Brain death | 223 | 163.09 ± 48.43 | 55 | 570 | |
| Live | 158 | 160.81 ± 44.57 | 100 | 532/67 | |
| HDL | 0.003 | ||||
| Brain death | 207 | 46.27 ± 19.23 | 6 | 259/5 | |
| Live | 132 | 54.63 ± 32.59 | 21 | 386 | |
| LDL | 0.15 | ||||
| Brain death | 207 | 91.07 ± 48.25 | 11 | 595 | |
| Live | 131 | 84 ± 34.49 | 28 | 286.5 |
Abbreviations: FBS, fasting blood sugar; TG, triglyceride; TC, total cholesterol; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
There was no significant difference in the use of immunosuppressant drugs between the 2 groups (brain dead or living; P > 0.05; Table 5).
| Drugs | Brain Death (N = 224) | Live (N = 158) | P-Value |
|---|---|---|---|
| Cyclosporine | 25 (6.5) | 7 (1.8) | 0.09 |
| Tacrolimus | 221 (57.9) | 153 (40.1) | 0.22 |
| Sirolimus | 44 (11.5) | 26 (6.8) | 0.42 |
| Cellcept | 197 (51.6) | 104 (27.2) | 0.07 |
| Prednisolone | 204 (53.4) | 136 (35.6) | 0.12 |
| Steroid pulse | 53 (13.9) | 29 (7.6) | 0.21 |
a Values are expressed as No. (%).
5. Discussion
In recent years, advances in surgical techniques and immunosuppressive drugs have increased the success of transplant surgery and the longevity of patients after liver transplant. The majority of liver transplant recipients need lifelong immunosuppression, mostly based on tacrolimus, cyclosporine, or sirolimus with or without steroids, which are associated with increasing risk of metabolic syndrome components (such as hypertension, diabetes mellitus, and hyperlipidemia) and increased risk of CVD (11). Thus, the success of liver transplants has increased dependence on the management and prevention of long-term problems. Obesity, excessive weight gain, and altered lipid profiles are recognized to be prevalent postoperative problems in liver transplant patients. Post-liver transplant diabetes is a well-known disorder associated with impaired graft tissue function, increased risk of infection, and CVD (12, 13).
A posttransplant metabolic disorder can lead to CVD and is associated with increased posttransplant mortality. In their study, Laish et al. reported a 59.1% prevalence of posttransplant metabolic disorders, which was found to be twice as high as the prevalence observed in the normal population (13). In our center, the rate of metabolic syndrome after liver transplant in children was 50.2% (7). Therefore, identifying the risk factors associated with this syndrome is recognized as an important issue in patients’ long life spans.
In Husing et al.'s study, hyperlipidemia was observed in 45% of patients with or without immunosuppressive drugs (14). In a combined pediatric and adult series at our facility, the rates of hypertriglyceridemia and hypercholesterolemia were 70% and 15.3%, respectively. Hypertriglyceridemia and hypercholesterolemia were not predicted by age, sex, BMI, or underlying liver disease. Patients receiving tacrolimus showed a considerably higher prevalence of posttransplant hypertriglyceridemia (P = 0.040) compared to those receiving cyclosporine; however, there was no significant link between the type of immune suppression and posttransplant hypercholesterolemia (15).
Tacrolimus was the most often prescribed immunosuppressive drug in the current study, followed by sirolimus, prednisolone, and mycophenolate. The use of immunosuppressive medications in the current study did not differ between genders, age groups, or kinds of transplants.
A recent study in pediatric liver transplant recipients showed that posttransplant metabolic syndrome and its components were common, as 28% of children and young adults were overweight or obese, 35% had pre-hypertension or hypertension, 44% had pre-diabetes, and 37% had low HDL (16). In the present study, prediabetes and diabetes were seen in 6.1% and 1.7% of the patients, respectively, and 65.6% of them had low HDL.
Pinto et al. evaluated the effect of diet on reducing lipid profiles in 53 patients with liver transplants, and their results showed that posttransplant TC, LDL, and TG profiles were significantly decreased by dietary intervention. The mean of each of these profiles was 160, 84.2, and 150 mg/dL for boys and 169, 95.8, and 123.5 mg/dL for girls, respectively (10).
In a study on 165 adult liver recipients, the lipid profiles were compared between the 2 groups of recipients (LD and DD), showing that living donor liver transplantation (LDLT) recipients had lower fasting glucose (4.85 vs. 7.21 mmol/L; P < 0.001) and TG (0.87 vs. 1.22 mmol/L; P = 0.016) but higher HDL (1.58 vs 1.39 mmol/L; P = 0.022). The authors concluded that LDLT recipients had better lipid profiles than deceased donor liver transplantation (DDLT) recipients (17).
The results of this study showed that the mean levels of FBS, TG, TC, and LDL were higher in patients with transplants from DD, and the mean HDL level was lower in these patients; however, these differences were significant only in TG and HDL profiles. The FBS and HDL levels increased and decreased with age, respectively. Also, the levels of TG and HDL factors were significantly correlated with the type of tissue graft used. Patients who received a transplanted organ from an LR donor have a significantly lower TG, higher HDL, and lower risk of CVD.
According to the results of this study, we can suggest that DDLT recipients need more closely monitored lipid profiles.
5.1. Study Limitations
First, the research scope was deliberately focused on comparing lipid profiles in pediatric liver transplant recipients from LR and DDs. Consequently, the study did not assess additional parameters, such as BMI Z scores, apolipoproteins (ApoAI, ApoB, and ApoE), LCAT, insulin resistance by HOMA-IR, and markers of oxidative stress and atherosclerosis, including glutathione (GSH), glutathione peroxidase (GPx), asymmetrical dimethyl arginine (ADMA), and oxidized LDL (ox-LDL). This decision was influenced by the need for a more specific exploration of factors directly relevant to the primary research objectives. Second, resource constraints posed limitations on the inclusion of certain assessments, particularly those involving more sophisticated procedures. Ethical considerations, especially in a pediatric population, further guided the exclusion of specific measurements. Despite these constraints, the study aimed to contribute valuable insights within its defined scope, and it is acknowledged that potential exists for future research to delve into the unexplored aspects highlighted.
5.2. Conclusions
Patients who received a liver from an LR donor have a significantly lower TG, higher HDL, and lower cardiovascular risk than patients who received a liver from a deceased-related donor.