1. Background
Vasculitis is characterized by inflammation within blood vessel walls, with clinical presentations varying based on the size and location of the affected vessels (1). Small vessel vasculitis, affecting arterioles, venules, and capillaries, can lead to bowel involvement, which is characterized by ulceration, bleeding, and the formation of strictures (2). Henoch-Schönlein purpura (HSP), the most common vasculitis in pediatric patients, is a type III hypersensitivity-mediated small vessel vasculitis. It is clinically characterized by non-thrombocytopenic palpable purpura, joint involvement, abdominal pain, and kidney involvement. Functional gastrointestinal disorders in HSP patients present a wide spectrum of heterogeneous diseases with elusive etiologies. Historically classified as non-organic disorders, emerging research highlights the presence of underlying low-grade inflammation (3). Gastrointestinal symptoms in HSP result from submucosal and subserosal bleeding, leading to fluid accumulation in the bowel wall (4). Gastrointestinal manifestations are common in children with HSP, occurring in approximately 50 - 75% of cases and ranging from abdominal pain and nausea/vomiting to gastrointestinal bleeding and intussusception. Notably, about 20% of pediatric patients may exhibit gastrointestinal symptoms up to two weeks before the onset of purpura (5). Gastrointestinal bleeding is observed in nearly one-third of pediatric cases, and intussusception occurs in approximately 1 - 5% of cases, predominantly in the ileoileal region (6). Small vessel vasculitis primarily affects intramuscular arteries, leading to focal, segmental ischemia and ulcers. Gastrointestinal symptoms associated with small vessel vasculitis range from mild abdominal pain to severe, potentially life-threatening complications, including intestinal perforation. Despite the high prevalence of gastrointestinal issues in patients with small vessel vasculitis, few studies have focused on this area. Consequently, the predictive factors for gastrointestinal complications in these patients remain inadequately determined and investigated.
Moreover, the existence of conflicting results regarding the prevalence of various gastrointestinal findings in small vessel vasculitis highlights the need for further research in this area. Therefore, the purpose of our study was to investigate the prevalence of gastrointestinal findings in patients with small vessel vasculitis and to identify associated predictive factors. Our initial hypothesis includes the following research questions:
Is there a correlation between gastrointestinal symptoms (such as abdominal pain, nausea, bleeding, diarrhea, vomiting, constipation, and lack of excretion) and demographic variables like age and sex, as well as clinical and laboratory findings, in patients with small vessel vasculitis?
Is there a connection between the type of vasculitis and the occurrence of gastrointestinal symptoms in these patients?
Additionally, is there a relationship between the duration of the disease and the presence of gastrointestinal symptoms in small vessel vasculitis patients?
2. Objectives
This research study was primarily undertaken to determine the prevalence of gastrointestinal manifestations in children with HSP and to identify predictive factors associated with these gastrointestinal manifestations.
3. Methods
3.1. Study Design
This retrospective cross-sectional study aimed primarily to investigate the association between gastrointestinal manifestations and predictive factors in children diagnosed with small vessel vasculitis.
3.2. Patient Selection
We included pediatric patients aged 1 to 16 years diagnosed with HSP at Mofid Children's Hospital in Tehran, Iran, from 2013 to 2022 in this cross-sectional study. We used a census method for sampling, selecting all available hospital records of children admitted with a confirmed diagnosis of small vessel vasculitis. Data retrieval was based on these hospital records. The diagnosis criteria for HSP adhered to the final classification criteria for IgA vasculitis (Henoch-Schönlein), as outlined in the Annals of the Rheumatic Diseases, Volume 69 (2010).
3.2.1. Purpura
Plus at least one of the following:
3.2.1.1. Abdominal Pain
Characterized as diffuse, acute, and colicky pain, it may include intussusception and gastrointestinal bleeding.
3.2.1.2. Histopathology
Evidence of leukocytoclastic vasculitis with predominant IgA deposits; or proliferative glomerulonephritis with predominant IgA deposits.
3.2.1.3. Arthritis or Arthralgias
Defined as arthritis (acute joint swelling or pain with limitation of motion) or arthralgia (acute joint pain without joint swelling or limitation of motion).
3.2.1.4. Renal Involvement
Manifested by proteinuria (> 0.3 g/24 hr or spot urine albumin to creatinine ratio > 30 mmol/mg), ≥ 2 + on dipstick, hematuria characterized by red cell casts, and urine sediment showing > 5 red cells per high-power field or red cell casts.
Patients were excluded from the study if their hospital admission file was incomplete or if they had a previous diagnosis of gastrointestinal bleeding at the time of admission. The study received approval from the relevant local ethics committee, and informed consent was obtained from the patients or their parents.
3.3. Data Collection
In our study, we included 295 cases diagnosed with HSP. We obtained demographic data such as age, sex, delivery type, weight, and height from hospital records. Additionally, clinical manifestations like fever, rash, abdominal distension, abdominal pain, abdominal tenderness, anorexia, nausea/vomiting, diarrhea, icterus, and blood in stool were recorded. Laboratory data, including ALT, AST, bilirubin, serum electrolytes, WBC, RBC, and occult blood, were also documented in the patients' admission files.
3.4. Statistical Analysis
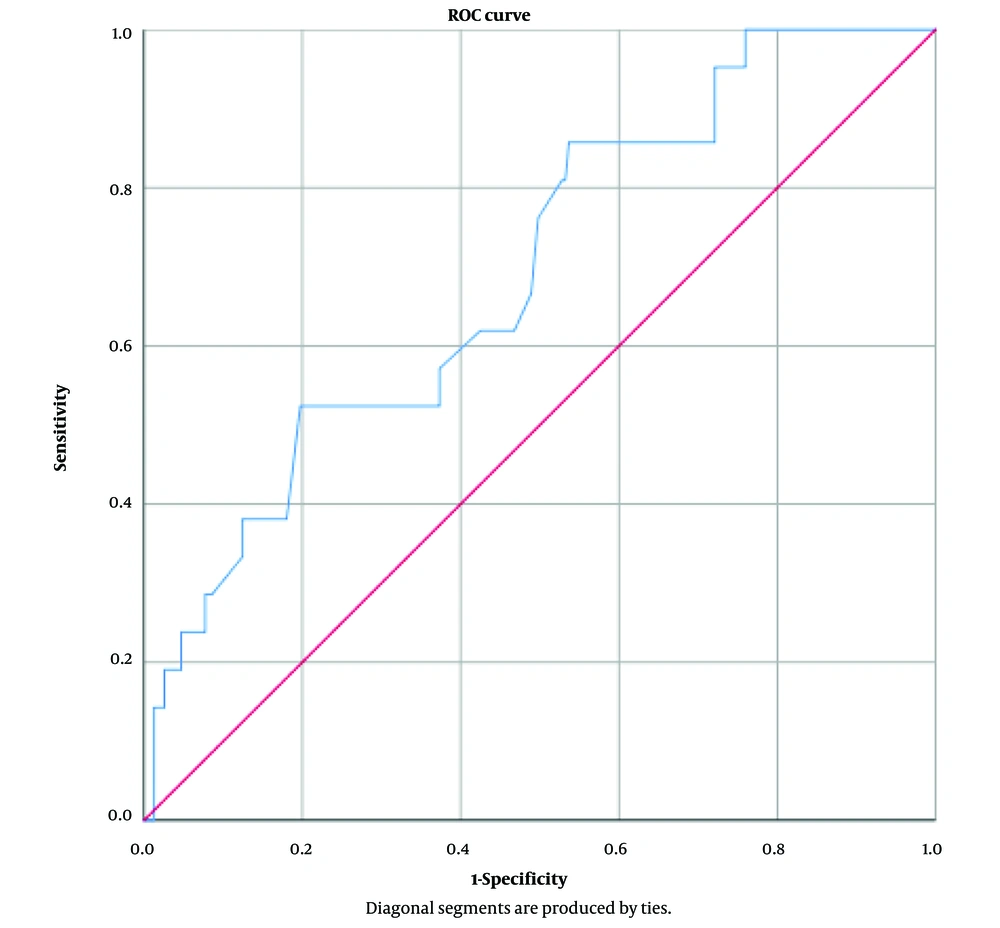
Statistical analysis was conducted using SPSS Statistics v.25.0 (Illinois, USA). Quantitative variables were expressed as mean ± SD, while qualitative variables were described in terms of frequency and percentage. The normality of variables was assessed using the Kolmogorov-Smirnov test. For comparing quantitative variables at 2 time points, the paired t-test was used for normally distributed variables, and the Wilcoxon test for non-parametric distributions. Repeated measure ANOVA was utilized for normally distributed variables compared at different time points; otherwise, the Chi-square test was applied. To select the best variables for inclusion in the model, we used a backward approach with a P-value < 0.25. Logistic regression was employed for statistical modeling and controlling confounding variables, setting a significance level at P < 0.05. The results of the logistic regression model were validated using the Hosmer-Lemeshow test and the ROC curve. The area under the ROC curve was determined to be 0.62, confirming the validity of the model.
3.5. Ethical Consideration
The study received ethics approval from the Ethics Committee of Shahid Beheshti Medical University. The study protocol adhered to the ethical guidelines outlined in the Declaration of Helsinki of 1875, as confirmed by the prior approval from the institution's Human Research Committee. Informed consent was obtained from all participants, either from the patients themselves or from their respective guardians.
4. Results
A total of 295 children diagnosed with HSP participated in our study. The group comprised 138 (46.77%) female and 157 (53.23%) male children. The average age was 5.3 ± 2.8 years for females and 6.2 ± 2.9 years for males, with a significant difference in mean age between males and females (P-value=0.01).
Clinical manifestations observed included fever, abdominal pain, nausea/vomiting, diarrhea, icterus, bloody stool, and anorexia. The most common clinical manifestations were anorexia (21%), followed by abdominal pain (14%), diarrhea (11%), nausea/vomiting (10%), and bloody stool (2%). None of the clinical manifestations showed significant differences in terms of sex.
Physical examination findings included abdominal distension and abdominal tenderness. Abdominal tenderness was observed in 88% of the children, and abdominal distension in 2%. No significant sex differences were found in these clinical findings.
Laboratory data, including ALT, AST, ALP, bilirubin (total and direct), amylase, lipase, and stool examination results (occult blood, WBC, RBC), are presented in Table 1, including mean values and standard deviations.
| Variables | Normal Range | Mean ± SD | Min | Max |
|---|---|---|---|---|
| AST (n = 255) | AST < 40 | 35.6 ± 50.8 a | 8 | 646 |
| ALT (n = 254) | ALT < 40 | 26.5 ± 58.0 | 1 | 652 |
| ALP (n = 235) | ALP < 135 > 45 | 422.4 ± 165.4 | 132 | 1212 |
| Tot Bili (n = 49) | total Bili > 5 | 3.2 ± 9.2 | 0.20 | 49 |
| Dir Bili (n = 48) | Bili > 1 | 1.3 ± 4.1 | 0.10 | 20 |
| Amylase (n = 33) | Amylase < 140 > 40 | 48.2 ± 18.7 | 11 | 98 |
| Lipase (n = 4) | Lipase < 160 | 16 ± 4.8 | 12 | 23 |
a Values are expressed as Mean ± SD.
Our descriptive data indicated that 13.7% of children exhibited elevated AST levels, 8.3% had elevated ALT levels (ALT > 40), 10.2% presented with hyperbilirubinemia (total bilirubin >5), and 10.4% had direct hyperbilirubinemia (bilirubin >1). Stool analysis results showed 21% positive for RBC, 24.85% positive for WBC, and 23.25% positive for occult blood. No significant statistical differences were observed in terms of sex.
Quantitative variables with normal distributions were compared using the paired t-test, while those with non-parametric distributions were compared using the Wilcoxon test. We employed a repeated measure ANOVA to compare variables at different time points when they exhibited a normal distribution. Based on the statistical analysis of laboratory data in relation to clinical manifestations, the meaningful correlations were as follows:
- Anorexia was more prevalent among patients with elevated AST levels compared to those with normal AST (P-value = 0.007).
- Bloody stool was more prevalent among patients with elevated ALT levels compared to those with normal ALT (P-value = 0.002).
- Bloody stool was more prevalent among patients with hyperbilirubinemia compared to those with normal bilirubin levels (P-value = 0.003).
- Bloody stool was more prevalent among patients with direct hyperbilirubinemia compared to those with normal direct bilirubin levels (P-value = 0.003).
A logistic regression model was used to assess factors related to increased ALT in patients with small vessel vasculitis. The Chi-square test determined the best variables for inclusion in the model using a backward approach with a P-value < 0.25. The results of the adjusted model showed that bloody stool was one of the effective factors in increasing ALT. A statistically significant relationship was observed between bloody stools and increased ALT levels, with an odds ratio (OR) of 7.98 (95% CI: 1.67 - 38.02) in patients with bloody stool.
Additionally, there was an association between abdominal pain and increased ALT levels, but it did not reach statistical significance (P-value = 0.23, OR = 0.26, 95% CI: 1.67 - 38.02). In patients with abdominal pain, the ALT level increased by an OR of 0.26. The analysis indicated a slight increase in the odds of ALT elevation with each year of age increase (crude OR: 1.12), but this association was not statistically significant (P = 0.14). Age did not have a significant association with ALT elevation, even after adjusting for other variables (adjusted OR: 1.09, P = 0.22).
The performance of the logistic regression model was assessed in terms of model fit using both the Hosmer-Lemeshow test and the ROC curve. The results of the Hosmer-Lemeshow test were non-significant, indicating a good fit of the model. Additionally, the ROC curve analysis yielded an area under the curve (AUC) of 0.62, thereby confirming the statistical acceptability of the model. Tables 3 and 4 refer to the results of Hosmer-Lemeshow test and ROC curve, respectively (Figure 1).
Table 2 presents the factors associated with increased ALT levels in patients with small vessel vasculitis. These factors include age, sex, fever, abdominal pain, nausea/vomiting, diarrhea, bloody stool, and anorexia.
| Variables | Crude | P-Value | Adjusted | P-Value |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| ALT≥40 | ||||
| Age | 1.12 (0.96 - 1.31) | 0.14 | 1.09 (0.94 - 1.27) | 0.22 |
| Sex | ||||
| Female | 1 | - | - | - |
| Male | 1.70 (0.63 - 4.55) | 0.28 | - | - |
| Fever | ||||
| No | 1 | - | - | - |
| Yes | 0.82 (0.17 - 3.98) | 0.81 | - | - |
| Abdominal pain | ||||
| No | 1 | - | 1 | |
| Yes | 0.28 (0.03 - 2.28) | 0.23 | 0.26 (0.03- 2.04) | 0.20 |
| Nausea/vomiting | ||||
| No | 1 | - | - | - |
| Yes | 0.39 (0.04 - 3.62) | 0.41 | - | - |
| Diarrhea | ||||
| No | 1 | - | ||
| Yes | 1.39 (0.35 - 5.52) | 0.63 | ||
| Bloody stool | ||||
| No | 1 | - | 1 | - |
| Yes | 9.81 (1.94 - 50.30) | 0.006 | 7.98 (1.67- 38.02) | 0.009 |
| Anorexia | ||||
| No | 1 | - | - | - |
| Yes | 0.88 (0.27 - 2.85) | 0.83 | - | - |
| Step | Chi-square | Df | Sig. |
|---|---|---|---|
| 1 | 12.073 | 8 | .148 |
The test result variable(s): Predicted probability has at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased.
5. Discussion
Despite the common occurrence of gastrointestinal manifestations and findings in small vessel vasculitis such as HSP, few studies focus on the predictive factors of GI complications. Our results indicate that anorexia was more common among patients with elevated AST levels, while bloody stool was more prevalent in patients with increased ALT levels. Additionally, hyperbilirubinemia (both total and direct) was more common compared to those with normal liver enzymes. Furthermore, bloody stool was more prevalent among patients with direct hyperbilirubinemia than in those with normal direct bilirubin levels.
The prospective study by Rosti et al. (7) on children with HSP concluded that hepatic enzymes were normal in 90% of patients at admission and showed a slight rise in 10% of patients during hospitalization. These enzyme levels returned to normal after 4 weeks, indicating a self-limited condition. Similar findings were observed in patients with acute hemorrhagic edema and infantile HSP syndrome (8).
Rosti et al. also noted that in HSP patients with elevated liver enzymes, factors such as infections, a history of hepatotoxic drug consumption, and recent heavy exercise should be ruled out (7). However, in our study, none of these factors were found in the medical histories of the recorded cases. It appears that further studies will be necessary to confirm these findings.
The frequency of elevated liver enzymes in our study was consistent with Rosti et al.'s findings, which observed self-remitting liver enzyme elevation in approximately 10% of children with HSP within a follow-up period of 2 - 4 weeks. However, their study did not investigate the predictability of these factors (7).
A 2022 study hypothesized HSP as a complication of hepatitis C, but no significant correlation was found between these findings and gastrointestinal manifestations such as bloody stool in children with HSP (9).
In 2017, D’Angelo et al. (10) conducted a study similar to ours, focusing on predicting gastrointestinal manifestations and complications in HSP patients. They considered the elevation of factor XIII as a predictor for gastrointestinal bleeding in HSP patients and also analyzed liver function tests. However, no statistically significant relationship was found (10). The variations in results could be attributed to factors such as differences in laboratory testing kits, implementation of various thresholds for defining liver enzyme elevation, and differences in the racial and age demographics of the study populations.
In 2014, Nagamori et al. (11) proposed a scoring system to predict severe gastrointestinal manifestations and GI bleeding in children with HSP. Their retrospective study analyzed patient data from 2009 to 2012 and incorporated serum WBC, absolute neutrophil count, serum albumin, serum potassium, D-dimer, and factor XIII activity into the scoring system. A score of 4 demonstrated a sensitivity of 90% and specificity of 80.6% for predicting GI bleeding in HSP patients. However, this scoring system did not include liver enzymes and bilirubin as contributing factors (11). This system, combined with our findings, may aid in predicting GI bleeding in children with HSP.
In 2020, Chao et al. (12) investigated common GI manifestations (abdominal pain and nausea) in 20 children aged 3 to 11 years with HSP. Their study revealed that 75% of patients exhibited elevated ALT levels, hepatomegaly, and increased gallbladder wall thickness, as observed in ultrasonographic studies (12). However, this study did not specifically address GI bleeding and anorexia, which our results indicate have significant correlations with liver enzyme levels and the presence of bloody stools. It is important to note that despite the hepatobiliary involvement observed in Chao et al.'s study (12), no relationship between bilirubin levels and clinical manifestations was found. This discrepancy may be due to differences in study populations (20 participants in their study compared to 295 in our), variations in laboratory kits and cut-off values, and differences in study design (prospective versus our retrospective approach).
In 2021, Guo et al. published research focusing on the clinical characteristics and risk factors of gastrointestinal perforation in children with small vessel vasculitis (13). Their retrospective study analyzed a cohort of 10,971 children with HSP from 2014 to 2018. Among these cases, 11 patients experienced gastric perforation, while 10 patients had intestinal perforation. The study emphasized the significance of early diagnosis and immediate management of HSP to prevent gastrointestinal perforation and the need for interventional surgery. It identified several factors as risk factors for GI bleeding, including hematochezia, renal involvement, abdominal pain, and prednisolone consumption greater than 2 mg/kg/day for at least 7 days.
Guo et al.'s study did not discuss laboratory data as risk factors for GI bleeding or gastrointestinal perforation, focusing instead on the importance of history taking, past medical history, and findings from physical examinations (13). In contrast, our study analyzed both clinical and paraclinical data, representing a specific strength of our research.
The findings from Tan et al.’s study, published in 2021, conflict with our research. Their study found that patients with IgA vasculitis who developed hyperbilirubinemia exhibited higher systolic blood pressure, increased proteinuria, lower serum albumin levels, and decreased hemoglobin levels. Consequently, this cohort experienced a less favorable prognosis compared to patients without hyperbilirubinemia (14). Additionally, they observed that lower serum bilirubin levels were associated with a higher rate of renal involvement and suggested that bilirubin may act as a protective cellular factor and internal antioxidant. In contrast, our research identified a higher prevalence of bloody stool, GI complications, and ultimately a poorer prognosis in children with hyperbilirubinemia. It is important to note that the study population in Tan et al.’s research was smaller and ethnically different (specifically Chinese) compared to ours, which limits the generalizability of their findings to diverse populations (14).
In 2020, Peak et al. (15) conducted a retrospective study analyzing 69 children with HSP, suggesting that stool calprotectin could be a relevant indicator for predicting GI manifestations (15). Similarly, Zhao et al. in 2019 focused on 135 children with HSP from northwest China, proposing that factors such as WBC count, neutrophil count, hemoglobin levels, D-dimer, PTT, and FDP might indicate GI complications (16). Notably, neither of these studies mentioned liver tests as prognostic factors, although they did examine various other laboratory data. It appears that multiple paraclinical parameters may provide valuable insights to clinicians as prognostic factors; however, further research and clinical trials are needed to identify the most reliable and informative prognostic factors.
However, identifying the most reliable and informative prognostic factor necessitates further research and clinical trials. Our analysis revealed that anorexia was more prevalent among patients with elevated AST, while bloody stool was more common in patients with increased ALT levels and hyperbilirubinemia (both total and direct), compared to those with normal liver enzymes. Furthermore, the prevalence of bloody stool was greater in patients with direct hyperbilirubinemia than in those with normal direct bilirubin levels.
To date, limited research has investigated the correlation between liver tests and bilirubin levels with gastrointestinal complications, such as bloody stool, in small vessel vasculitis. This study explored the associations between ALT elevation and various factors, including age, sex, fever, abdominal pain, nausea/vomiting, diarrhea, bloody stool, and anorexia. We calculated both crude and adjusted odds ratios (ORs) to assess potential confounding variables. The results suggest that age may not be a significant factor contributing to ALT elevation in patients with small vessel vasculitis. The crude OR for males compared to females indicated higher odds of ALT elevation (crude OR: 1.70), but this association was not statistically significant (P = 0.28). Further research is needed to investigate the potential correlation between sex and ALT elevation in small vessel vasculitis.
Among the other factors analyzed, the presence of bloody stool demonstrated a statistically significant association with ALT elevation. The crude OR for bloody stool was 9.81, indicating significantly higher odds of ALT elevation in patients with this symptom (P = 0.006). This association remained significant even after adjusting for other variables (adjusted OR: 7.98, P = 0.009). These findings indicate that bloody stool may be an important indicator of ALT elevation in patients with small vessel vasculitis. These factors can serve as prognostic indicators for ALT elevation, aiding in the identification of patients at higher risk and enabling closer monitoring of their liver function.
Given the limitations of a small sample size and challenges in patient cooperation encountered in our study, future research should prioritize including larger sample sizes and patients with a clinically acceptable positive predictive value. Consequently, further studies are essential to clarify these associations and identify definitive predictive factors.
It is important to acknowledge the inherent limitations of our study, which primarily arise from the retrospective extraction of data from patient records. This approach limited our ability to conduct comprehensive assessments, including ultrasonography and endoscopy, and resulted in incomplete evaluation of clinical symptoms in some cases, particularly regarding kidney, joint, and CNS involvement. As a result, this study focuses mainly on evaluating gastrointestinal manifestations and their associated prognostic factors.
5.1. Conclusions
This study explored the clinical manifestations and laboratory findings in children diagnosed with small vessel vasculitis, aiming to identify factors associated with elevated ALT levels. Our findings highlighted that the most prevalent clinical manifestations were anorexia (21%), followed by abdominal pain (14%), diarrhea (11%), nausea/vomiting (10%), and bloody stool (2%). Physical examination findings included abdominal distension in 88% of patients and abdominal tenderness in 2%. Notably, only bloody stool demonstrated a significant association with ALT elevation. Moreover, logistic regression analysis identified bloody stool as a significant factor contributing to the elevation of ALT. These results underscore the importance of vigilant liver function monitoring in small vessel vasculitis patients presenting with bloody stool. However, further research is necessary to understand the underlying mechanisms and causative factors leading to ALT elevation in these patients.