1. Background
Vasculitides are characterized by inflammatory processes affecting the blood vessel wall, with clinical presentations depending on their size and location (1). Medium vessel vasculitis primarily involves the main visceral arteries and veins along with their initial branches (2).
Kawasaki disease, the second most common childhood medium-sized vasculitis, is marked by fever, erythema of the lips and oral mucosa, bilateral non-exudative conjunctivitis, extremity changes, rash, and cervical lymphadenopathy (2). Coronary artery involvement occurs in 20% of Kawasaki cases and can lead to myocardial infarction or sudden death (3).
Gastrointestinal complications of Kawasaki disease encompass a wide range of manifestations such as pseudo-obstruction, gallbladder hydrops, pancreatitis, duodenitis, and duodenal perforation. Associated gastrointestinal symptoms include abdominal pain, diarrhea, nausea, and vomiting (4).
Diagnosing and managing Kawasaki disease can be challenging due to gastrointestinal presentations, especially if they precede characteristic clinical manifestations of Kawasaki, increasing the risk of cardiac complications from delayed treatment. This delay may also lead to unnecessary surgical interventions and invasive procedures (5).
Therefore, there is a crucial need to identify predictive factors of gastrointestinal manifestations in Kawasaki patients. These factors will aid clinicians in interpreting gastrointestinal manifestations in the context of atypical Kawasaki disease.
2. Objectives
This study aims to assess the frequency of gastrointestinal manifestations in Kawasaki disease and identify associated predictive factors in children, aiming to prevent unnecessary invasive interventions.
3. Methods
3.1. Study Design
We devised the basic framework for this cross-sectional study with the primary objective of assessing gastrointestinal manifestations and associated prognostic factors in children with medium vessel vasculitis.
3.2. Patient Selection
This retrospective study included all children aged 1 to 16 years diagnosed with Kawasaki disease, gathered from Mofid Children's Hospital, Tehran, Iran, between 2013 and 2022. Data were retrieved from hospital records. Patients were excluded if their hospital admission files were incomplete or if they had known pre-existing gastrointestinal diseases. The study received approval from the relevant local ethics committee.
3.3. Data Collection
A total of 359 cases of Kawasaki disease were included in our study. Demographic information (age, sex, mode of delivery, weight, height) was extracted from hospital records. Additionally, clinical features (abdominal pain, nausea/vomiting, diarrhea, jaundice, bloody stool, anorexia, hepatomegaly, splenomegaly, abdominal tenderness, abdominal distension) and laboratory data (ALT, AST, alkaline phosphatase, bilirubin, amylase, stool examination (WBC, RBC, occult blood)) were recorded using a pre-prepared questionnaire. Patients were categorized as having high AST, ALT, and bilirubin levels if these values were equal to or greater than 40, 40, and 1, respectively.
3.4. Statistical Analysis
Statistical analysis was conducted using SPSS Statistics 23.0 (Illinois, USA). Quantitative variables were presented as mean ± SD. A P-value of < 0.05 was considered statistically significant for all analyses.
3.5. Ethical Considerations
Ethics approval was obtained from the ethics committee of Shahid Beheshti Medical University. The study protocol adhered completely to the ethical guidelines of the 1875 Declaration of Helsinki, as evidenced by prior approval from the institution's human research committee.
4. Results
A total of 359 children with Kawasaki disease were included in our study, comprising 153 (42.6%) females and 206 (57.4%) males. The mean age was 2.7 ± 2.5 years for females and 2.8 ± 2.7 years for males. There was no statistically significant difference in mean age between males and females (P-value = 0.56).
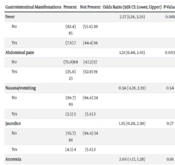
Clinical manifestations observed included abdominal pain, nausea/vomiting, diarrhea, jaundice, bloody stool, and anorexia. Abdominal pain was the most prevalent clinical manifestation (39%), followed by anorexia (11%), nausea/vomiting (4%), diarrhea (4%), jaundice (1%), and bloody stool (0.2%). There were no significant differences in clinical manifestations between genders.
Physical examination findings included hepatomegaly, splenomegaly, abdominal distension, and abdominal tenderness. Abdominal tenderness was noted in 8% of children, and splenomegaly in 2%. No statistically significant differences were found between genders. None of the patients exhibited abdominal distension or hepatomegaly.
Laboratory data, including ALT, AST, alkaline phosphatase, amylase, bilirubin (total and direct), and stool examination (OB, WBC, and RBC), were assessed for all children. AST elevation (> 40) was observed in 38.1% of children, while ALT elevation (> 40) was seen in 30.2%. Hyperbilirubinemia (total bilirubin > 5) was present in 4.7% of cases, and direct hyperbilirubinemia (direct bilirubin > 1) in 10.5%. Stool analysis revealed 14% positive for RBC, 28% positive for WBC, and 9% positive for occult blood. There were no significant gender differences in laboratory findings.
It is important to note that this study provides only a snapshot of the disease at a single point in time and cannot establish causality. However, based on the statistical analysis of laboratory data regarding clinical manifestations and findings, the following meaningful correlations were observed (Tables 1 - 3):
- The frequency of anorexia, abdominal pain, and nausea/vomiting was associated with AST elevation (P-values in order of manifestations mentioned above: 0.001, 0.001, and 0.007).
- The frequency of anorexia and nausea/vomiting was associated with ALT elevation (P-values in order of manifestations mentioned above: 0.001 and 0.001).
- The frequency of abdominal pain was associated with hyperbilirubinemia (P-value = 0.003).
- The frequency of abdominal pain was associated with positive WBC in stool analysis (P-value = 0.003).
- The frequency of abdominal pain was associated with positive RBC in stool analysis (P-value = 0.001).
| Gastrointestinal Manifestations | AST | ALT | ||||||
|---|---|---|---|---|---|---|---|---|
| < 40 | ≤ 40 | Odds Ratio (95% CI: Lower, Upper) | P-Value | < 40 | ≤ 40 | Odds Ratio (95% CI: Lower, Upper) | P-Value | |
| Fever | 0.61 (-0.06, 1.27) | 0.07 | 0.66 (-0.02, 1.34) | 0.05 | ||||
| No | (91.1) 194 | (84.7) 111 | (90.8) 216 | (83.5) 86 | ||||
| Yes | (8.9) 19 | (15.3) 20 | (9.2) 22 | (16.5) 17 | ||||
| Abdominal pain | 0.81 (0.30, 1.31) | 0.001 | 0.93 (0.41, 1.45) | 0.001> | ||||
| No | (82.2) 175 | (67.2) 88 | (81.9) 195 | (64.1) 66 | ||||
| Yes | (17.8) 38 | (32.8) 43 | (18.1) 43 | (35.9) 37 | ||||
| Nausea/vomiting | 1.34(0.26, 2.41) | 0.01 | 1.71 (0.63, 2.80) | 0.001 | ||||
| No | (97.7) 208 | (91.6) 120 | (97.9) 233 | (89.3) 92 | ||||
| Yes | (2.3) 5 | (8.4) 11 | (2.1) 5 | (10.7) 11 | ||||
| Jaundice | 1.19 (-1.22, 3.6) | 0.30 | 1.54 (-0.87, 3.96)) | 0.16 | ||||
| No | (99.5) 212 | (98.5) 129 | (99.6) 237 | (98.1) 101 | ||||
| Yes | (0.5) 1 | (1.5) 2 | (0.4) 1 | (1.9) 2 | ||||
| Anorexia | 0.89 (0.23, 1.54) | 0.007 | 1.20 (0.54, 1.86) | 0.001> | ||||
| No | (91.5) 195 | (81.7) 107 | (92.0) 219 | (77.7) 80 | ||||
| Yes | (8.5) 18 | (18.3) 24 | (8.0) 19 | (22.3) 23 | ||||
| Gastrointestinal Manifestations | Direct Bilirubin | |||
|---|---|---|---|---|
| < 1 | ≤ 1 | Odds Ratio (95% CI: Lower, Upper) | P-Value | |
| Fever | 2.56 (1.17, 3.95) | < 0.001 | ||
| No | (91.5) 86 | (45.5) 5 | ||
| Yes | (8.5) 8 | (54.5) 6 | ||
| Abdominal pain | 1.89 (0.49, 3.29) | 0.003 | ||
| No | (71.3) 67 | (27.3) 3 | ||
| Yes | (28.7) 27 | (72.7) 8 | ||
| Nausea/vomiting | 2.43 (0.68, 4.19) | 0.001 | ||
| No | (96.8) 91 | (72.7) 8 | ||
| Yes | (3.2) 3 | (27.3) 3 | ||
| Jaundice | 2.33 (0.25, 4.40) | 0.008 | ||
| No | (97.9) 92 | (81.8) 9 | ||
| Yes | (2.1) 2 | (18.2) 2 | ||
| Anorexia | 1.04 (-0.43, 2.50) | 0.15 | ||
| No | (88.3)83 | (72.7) 8 | ||
| Yes | (11.7) 11 | (27.3) 3 | ||
| Gastrointestinal Manifestations | Stool WBC | Stool RBC | ||||||
|---|---|---|---|---|---|---|---|---|
| Present | Not Present | Odds Ratio (95% CI: Lower, Upper) | P-Value | Present | Not Present | Odds Ratio (95% CI: Lower, Upper) | P-Value | |
| Fever | 2.27 (1.26, 3.29) | 0.001 | 1.23 (0.16, 2.30) | 0.01 | ||||
| No | (92.4) 85 | (55.6) 20 | (85.5) 94 | (63.2) 12 | ||||
| Yes | (7.6) 7 | (44.4) 16 | (14.5) 16 | (36.8) 7 | ||||
| Abdominal pain | 1.21 (0.40, 2.01) | 0.003 | 0.99 (0.00, 1.99) | 0.04 | ||||
| No | (75.0)69 | (47.2) 17 | (70.9) 78 | (47.4) 9 | ||||
| Yes | (25.0) 23 | (52.8) 19 | (29.1) 32 | (52.6) 10 | ||||
| Nausea/vomiting | 0.56 (-1.28, 2.39) | 0.54 | -0.710 (-3.65, 2.23) | 0.34 | ||||
| No | (96.7) 89 | (94.4) 34 | (95.5) 105 | (100) 19 | ||||
| Yes | (3.3) 3 | (5.6) 2 | (4.5) 5 | - | ||||
| Jaundice | 1.25 (0.20, 2.30) | 0.77 | 0.63(-2.61, 3.86) | 0.89 | ||||
| No | (95.7) 88 | (94.4) 34 | (95.5) 105 | (94.7) 18 | ||||
| Yes | (4.3) 4 | (5.6) 2 | (4.5) 5 | (5.3) 1 | ||||
| Anorexia | 2.06 (-1.17, 5.28) | 0.10 | 1.07 (-0.11, 2.26) | 0.67 | ||||
| No | (100)92 | (97.2) 35 | (99.1)109 | (100) 19 | ||||
| Yes | - | (2.8) 1 | (0.9) 1 | - | ||||
5. Discussion
Despite the significance of early diagnosis and treatment in children with Kawasaki disease, particularly those presenting with gastrointestinal manifestations, there is limited literature focusing on predicting factors for GI complications in Kawasaki patients.
Our results indicate a higher prevalence of anorexia, abdominal pain, and nausea/vomiting among patients with elevated AST levels. Similarly, the prevalence of anorexia and nausea/vomiting was higher among patients with elevated ALT levels. Additionally, patients with positive RBC and WBC in stool analysis had a higher prevalence of abdominal pain compared to those with normal S/E.
Eladawy et al. published an article in 2013, examining 7 cases of Kawasaki disease among 118 cases of abdominal pain. They found that 86% of patients had abnormalities in aminotransferase levels (6). In our study, abdominal pain was also the most common clinical manifestation, with 38.1% of patients exhibiting elevated AST levels and 30.2% showing elevated ALT levels. The number of Kawasaki cases of Eladawy research was less than ours (7 versus 359). So, it seems that the frequency of clinical findings and laboratory abnormalities in our research is more reliable and generalizable.
Fabi et al. studied 302 children with Kawasaki disease, classifying them into two groups based on the presence or absence of gastrointestinal findings. They concluded that gastrointestinal manifestations were an independent risk factor for aortic aneurysms in Kawasaki patients (7). While we did not analyze cardiac complications in our study, we focused on the frequency of gastrointestinal manifestations. Our results suggest that elevated hepatic enzymes could serve as predictive indicators for abdominal pain, nausea/vomiting, and anorexia. This finding is particularly valuable in atypical Kawasaki cases to minimize unnecessary invasive interventions.
A similar topic was explored in a cross-sectional study by Nasri et al. in 2020. They investigated the frequency of gastrointestinal presentations during the acute phase of Kawasaki disease retrospectively. According to their findings, nausea was the most common gastrointestinal manifestation (28.9%), followed by abdominal pain (17.4%) and diarrhea (16.9%). Elevated AST and ALT levels were observed in 52.37% and 46.26% of cases, respectively (8). Discrepancies in the frequencies of gastrointestinal presentations and laboratory data between their study and ours could be attributed to differences in study populations, examining physicians, patient compliance, and variations in laboratory kits.
In a retrospective study conducted by Lee et al. in 2022, it was noted that many atypical Kawasaki patients exhibited gastrointestinal manifestations and findings such as abdominal pain, distension, diarrhea, nausea, jaundice, pancreatitis, gallbladder hydrops, pseudo-obstruction, hematemesis, and paralytic ileus. However, our patients did not present with hydrops, pseudo-obstruction, ileus, or pancreatitis, which may be attributed to the absence of ultrasonographic data (9). In another study by Sadeghi et al., gallbladder issues were reported in 38% of cases, with hydrops observed in nearly 1% (10). Additionally, rare manifestations like pseudo-obstruction and hematemesis due to hemorrhagic duodenitis were not encountered in our research (8). It is important to note that abdominal pain, nausea, and hematemesis can also be potential presentations of Kawasaki disease, potentially leading to delayed diagnosis (10). Another aspect of Lee W et al.'s study focused on alterations in liver enzymes, which were found to commonly occur during Kawasaki disease. Similarly, two other studies have also noted increases in ALT and AST levels during Kawasaki disease (37%) (11, 12).
Kuo et al. have suggested that elevated ALT and AST levels could serve as predictors for atypical Kawasaki disease and have recommended liver enzyme testing in patients susceptible to Kawasaki disease with atypical manifestations. This article also mentioned hypoalbuminemia as a predictive indicator, which was not assessed in our study (12).
In a retrospective study conducted by Huang et al. in 2020, 10,367 Kawasaki patients were evaluated with the main objective of identifying predictive factors. According to their analyses, risk predictors included monocyte count, serum prealbumin level, GGT (Gamma Glutamyl Transferase), and the AST/ALT ratio. Elevated serum GGT and ALT were observed in 50% of their cases (13).
The prevalence of elevated liver transaminases in Kawasaki children was estimated at 46% by Soleimani et al. in a cross-sectional study spanning from 2006 to 2013. They concluded that Kawasaki disease should be considered in infants and children with elevated liver enzymes, even if they lack the classic diagnostic criteria of Kawasaki. These findings are consistent with ours (14). Another study has also indicated that elevated levels of ALT, AST, and GGT, along with low serum albumin levels, indicate severe liver inflammation and are directly associated with Kawasaki disease (15).
Cheng et al. assessed hyperbilirubinemia among Kawasaki children in 2020. They found that 37.7% of their cases had hyperbilirubinemia, with 70.83% of those cases exhibiting direct hyperbilirubinemia. In our study, we calculated a 10.5% prevalence of hyperbilirubinemia. Differences in these figures could be attributed to racial, geographic, and demographic variations between the two populations. Our analysis revealed a correlation between hyperbilirubinemia and abdominal pain. However, Cheng et al. concluded that hyperbilirubinemia is associated with leukocytosis, elevated AST and ALT levels, as well as an increase in ESR and CRP, all of which are indicative of severe liver damage and biliary obstruction in the acute phase of Kawasaki disease. They also highlighted the impact of IL-1, IL-6, and TNF-α on the elevation of ALT and AST levels, as well as immunologic hepatocellular damage during the inflammatory phase of Kawasaki disease (16).
Gottesman et al. introduced some new factors not previously discussed in recent articles. They explored the viral etiology of Kawasaki disease and its impact on liver and biliary duct inflammation, leading to direct hyperbilirubinemia. Additionally, they highlighted the significance of self-prescribed anti-febrile and antibiotic medications. According to their findings, children with a history of self-medication had a higher prevalence of direct hyperbilirubinemia. However, we did not inquire about previous medication use in our cases (17).
Inflammatory indices, such as WBC, platelet (Plt), ESR, and CRP are elevated in the acute phase of Kawasaki disease, particularly in patients with direct hyperbilirubinemia compared to those without it. Hyperbilirubinemia is primarily caused by hepatocyte lysis and cholestasis. Moreover, the fever and acute inflammatory phase of Kawasaki disease can induce hemolysis, contributing to hyperbilirubinemia (16, 18). Some studies have discussed the inhibitory effect of Hepcidin peptide during the acute phase of Kawasaki disease, leading to decreased duodenal absorption and macrophage-mediated iron storage in hepatic cells, resulting in anemia. According to these discussions, hyperbilirubinemia and anemia are two important indicators of the severity of the acute inflammatory phase of Kawasaki disease (19, 20).
We conducted an analysis of stool samples from cases in the acute phase of the disease and evaluated its correlation with clinical manifestations. Few studies have examined the predictive value of stool analysis. Our results showed a higher prevalence of abdominal pain in patients with stool samples positive for WBC and RBC.
An article published in 2021 concluded a direct correlation between bloody stool and acute myocarditis in Kawasaki disease. Since the occurrence of myocarditis indicates the severity of Kawasaki disease, positive RBC and WBC in stool analysis are predictive factors for severe Kawasaki disease, which aligns with our results (4).
A case report introduced intussusception as a systemic vasculitis in Kawasaki patients, manifesting with clinical symptoms of bloody stool and abdominal pain. Patients might undergo radiologic or surgical interventions in addition to the classic IVIG (Intravenous immunoglobulin) therapy for Kawasaki disease. The article highlighted the similarity in etiologies (viral) and age range between Kawasaki disease and intussusception and emphasized that both conditions may present with similar manifestations such as fever, abdominal pain, and bloody stool (21). However, there are limited articles discussing this index, and further evaluations are necessary to confirm this result.
This study has potential limitations. It is a retrospective study, and it is not possible to determine if the association of factors and gastrointestinal manifestations is causal. Additionally, it is a single-center study, limiting the generalizability of the conclusions drawn. Furthermore, the lack of a control group in this study makes the observed associations prone to bias. Further research is needed to explore the relationship between liver enzyme alterations and other clinical outcomes, such as cardiac complications.
The findings of this study align with previous research indicating gastrointestinal symptoms as a common manifestation of Kawasaki disease, underscoring the importance of evaluating stool samples in suspected cases. However, further investigation is necessary to explore the relationship between stool analysis and other clinical outcomes, such as disease severity and treatment response. Additionally, investigating the potential diagnostic utility of stool analysis, particularly in cases with atypical presentations or delayed diagnosis, could be valuable. Overall, our study offers initial evidence of the clinical relevance of stool analysis in Kawasaki disease, warranting further exploration in future research endeavors.
To date, there have been limited studies examining the relationship between liver tests and bilirubin levels with gastrointestinal complications like bloody stool in medium-sized vessel vasculitis. Therefore, additional research is warranted to elucidate these associations and identify definitive prognostic factors.
5.1. Conclusions
Based on our findings, elevated levels of AST and ALT, hyperbilirubinemia, and positive stool RBC and WBC are potential prognostic factors for the gastrointestinal manifestations of Kawasaki disease. To validate these findings, further research involving larger populations and long-term follow-up is recommended.