1. Background
In the late 1800s, researchers made initial practical observations of drug interactions. However, it wasn't until the 1940s, when the first reports of drug interaction occurrences were published in academic circles, that the issue began to be taken seriously (1). Drug-drug interactions are one of the common causes of unwanted drug-related problems (2-7). These interactions can result in treatment failure and drug complications, which can exacerbate the clinical course and severity of the disease, prolong hospitalization, and impose substantial costs on the healthcare system (8, 9). Some studies have estimated that as much as 3% of hospital admissions are due to drug-drug interactions (6).
It is important to be aware of drug-drug interactions (DDIs), as they are one of the most significant drug-related problems. Interactions between drugs occur when the effect of a drug is altered by another drug, liquid, food, or other chemical factors, which can be detrimental or sometimes beneficial (10). Drug interactions can happen in various forms, such as pharmacodynamic interactions, where the effects of different agents interact to enhance or reduce drug effects. Similarly, pharmacokinetic interactions may affect the blood levels of specific agents by either increasing or decreasing them (7).
The prescription of multiple medications simultaneously for a patient's disease is necessary, which could lead to the emergence of drug-drug interactions (11). An increase in the number of administered medications could lead to an increase in the prevalence of DDIs (12). Furthermore, the most prevalent drug-related complications among patients are adverse drug reactions (ADRs). ADRs are common, expensive, and may have fatal consequences (9, 13). This risk is increased by the high number and dose of prescribed medications (14).
2. Objectives
Irrational drug prescribing imposes significant costs on patients and the healthcare system, as well as increases the number of side effects and drug interactions. Due to differences in pharmacokinetic parameters and increased sensitivity to drug interactions and medication side effects in the pediatric population, greater attention is required than in the adult population (15). Therefore, we have decided to evaluate these issues by examining pediatric outpatients.
3. Methods
3.1. Study Design and Participants
The population under study encompasses all pediatric outpatients who satisfied the inclusion criteria and were referred to the clinic or emergency department of Bandar Abbas Children's Hospital and received their medication from the hospital pharmacy. This investigation was conducted on patients referred to the Children's Hospital affiliated with the Hormozgan Faculty of Pharmacy and Medical Sciences between October 2022 and the end of May 2023.
3.2. Ethical Clearance
The study was conducted in accordance with the Declaration of Helsinki, and the board of ethics committee approved the study with the code IR.HUMS.REC.1401.220.
3.3. Inclusion and Exclusion Criteria
Patients under 18 years of age who did not require hospitalization were included in this study. The patients obtained their medications directly from the hospital pharmacy. The patient's legal guardian was provided with an explanation of the research plan and subsequently gave written informed consent for the patient's participation in the study. Patients needed to receive at least two medications in their prescription, excluding only supplements or herbal medicines. To ensure the study's accuracy, we utilized criteria to exclude patients who were either ineligible or unwilling to participate.
3.4. Data Collection Procedures
The data were collected using a pre-designed form that included patient demographics, clinical information, and medication details in individual case report forms. The relevant data were collected through self-reports provided by parents or legal guardians. Then, the prescriptions for the patients' medications were assessed. Using the online Lexi-Interact software, interactions were verified. This database classifies interactions into categories A, B, C, D, and X. In this study, clinically important interactions, including interactions of categories C, D, and X, were evaluated. In addition, the number, category, severity, and reliability constant of drug-drug interactions for these patients were assessed.
The study did not analyze drug interactions that occur when two medications or dosage forms are chemically or physically incompatible when combined. This was beyond the scope of the study, and the Lexi-Interact software did not support the evaluation of this type of interaction. The anatomical therapeutic classification system and Defined Daily Dose Index were used to determine the drug classes implicated in interactions.
3.5. Data Analysis
This research data was analyzed using SPSS version 25 (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.). The Kolmogorov-Smirnov test and normality diagram were employed to assess the normality of continuous variables. Variables with a normal distribution were represented by the mean ± standard deviation, while those with a non-normal distribution were represented by the median and interquartile range. Frequency and percentages were assigned to qualitative variables. For continuous variables, parametric and non-parametric data were compared using the Mann-Whitney U and Independent t-test. The potential relationship between categorical variables was examined using the chi-square or Fisher's exact test. When more than 20% of the variables had an expected frequency of less than 5, Fisher's exact test was conducted. In addition, prescription errors, including prescribing more than the allowed limit, prescribing less than the allowed limit, or providing incorrect administering instructions, are listed separately below.
4. Results
A total of 1011 patients were included in this study, of which 46.1% were female and 53.9% were male. The mean age was 4.52 ± 0.106 years. The average weight of the patients was 16.63 ± 0.307 kg. Furthermore, general practitioners (GPs) visited 710 (70%) of the patients, while 301 (30%) were visited by specialist and subspecialist practitioners (Table 1).
| Variables | With Interaction; n = 270 | Without Interaction; n = 741 | P-Value |
|---|---|---|---|
| Physician | 0.9 | ||
| Specialist and sub-specialist | 81 (30.0) | 220 (29.7) | |
| General practitioner | 189 (70.0) | 521 (70.3) | |
| Gender | 0.8 | ||
| Male | 123 (45.5) | 343 (46.3) | |
| Female | 147 (54.5) | 398 (53.7) |
Frequency of the Prescribed Drugs Based on the Gender, Physicians, and Interactions a
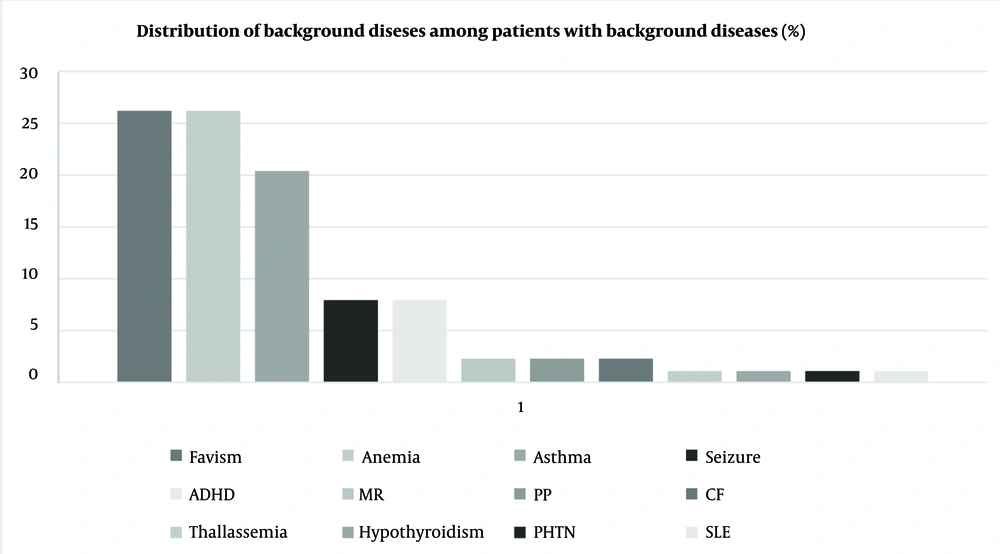
As reported in Figure 1, 96 patients (9.5%) had underlying diseases. Glucose-6-phosphate dehydrogenase (G6PD) deficiency, anemia, and asthma were the most common underlying conditions (26.2%, 26.2%, and 20.4%, respectively). Among the total population, 79 (7.8%) had at least one type of allergy, categorized into drug, food, seasonal, and animal allergies, with seasonal allergy being the most common type. Notably, among 20 patients with drug allergies, eight were allergic to antibiotics.
In addition, 972 patients (96.1%) had no history of taking any medications, while 39 patients (3.9%) had a history of taking at least one medication. The most commonly used medications among the patients were salbutamol oral inhalers and fluticasone oral inhalers. During physicians' visits, the most common complaints were cough (509, 24.8%), fever (447, 21.8%), and rhinorrhea (338, 16.5%).
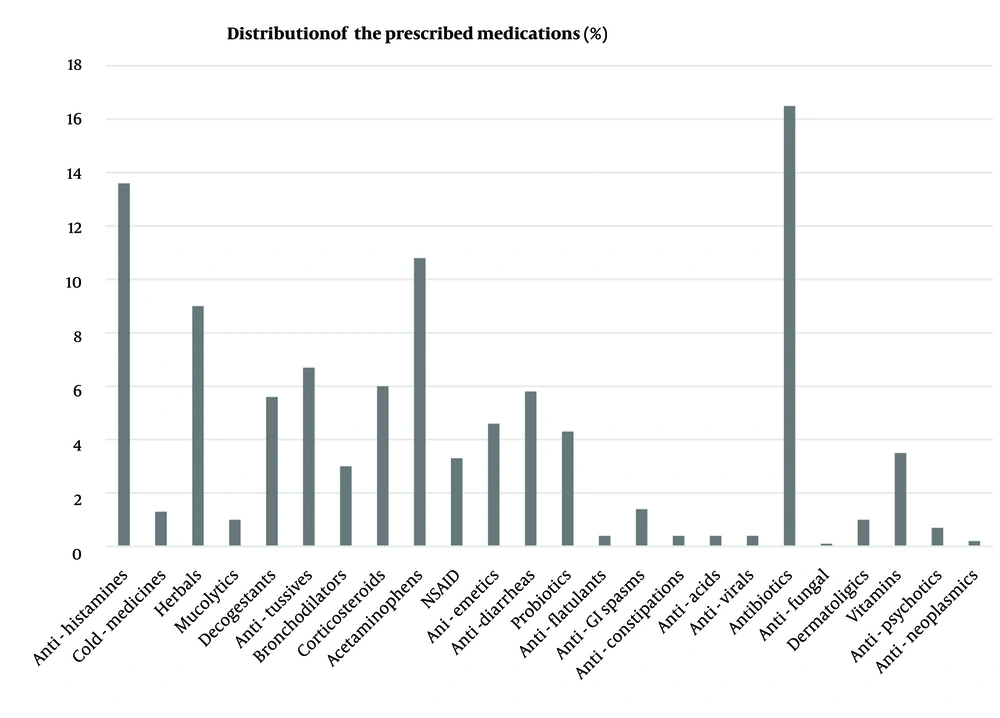
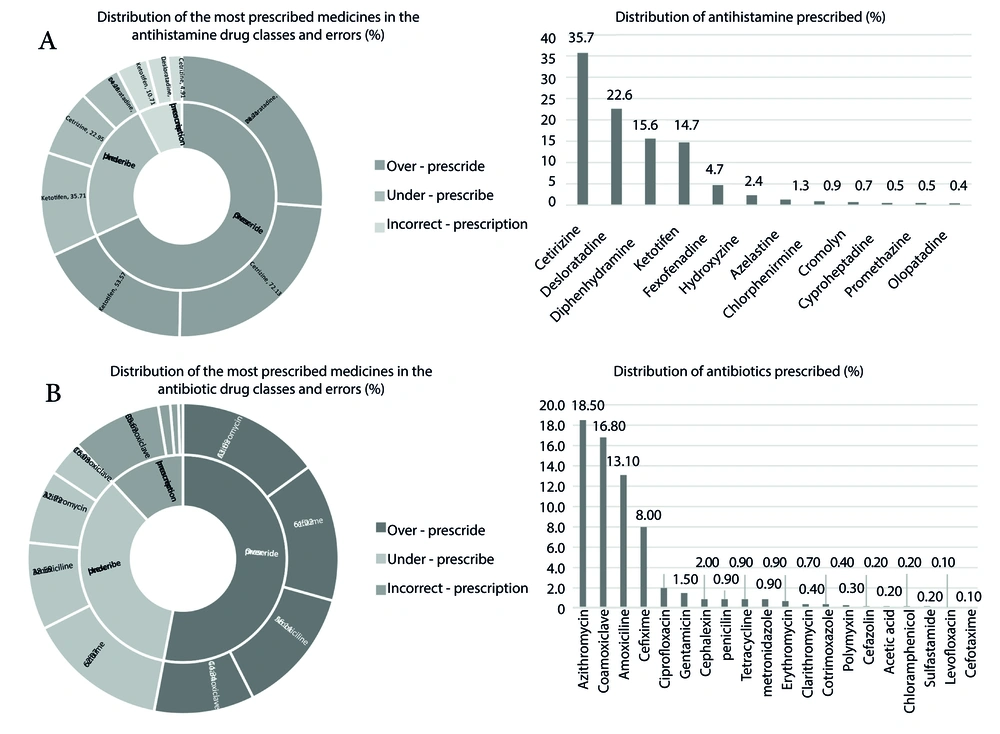
Antibiotics were the most commonly prescribed drug class, with azithromycin suspension being the most frequently prescribed individual drug at 18.5% (Figure 2). The antihistamine drug class followed antibiotics as the second most frequently used medication, with cetirizine being the most commonly prescribed antihistamine and olopatadine ophthalmic drops being the least common (35.7% vs. 0.4%). Among herbal medicines prescribed by physicians, pelargonium syrup (Pelargin®, Pars Gita Daru Co. Iran) was routinely prescribed for cold-related complaints (Figure 3).
After evaluating the prescribed medications, 309 drug-drug interactions (DDIs) were identified in 270 patients. Among all participants, 26.7% had at least one clinically significant DDI in their prescription. It is noteworthy that some prescriptions contained more than one drug-drug interaction. Additionally, the risk rating of C was the most common, followed by X and D (87.7%, 7.4%, and 4.8%, respectively). Table 2 shows the drugs with the most threatening risk rating (X) in detail. The most frequently reported interactions in category D were diphenhydramine/hydroxyzine, while diphenhydramine/cetirizine was the most common in category C. These two interactions could lead to suppression of the Central Nervous System (CNS). Table 3 provides details on DDIs with clinical significance.
| Drug 1 | Drug 2 | Risk Rating | Interaction’s Severity | Reliability | Frequency |
|---|---|---|---|---|---|
| Category X | |||||
| Azelastine-fluticasone | Desloratadine | X | Moderate | Fair | 2 (0.6) |
| Cetirizine | Salbutamol-Ipratropium | X | Moderate | Fair | 3 (0.9) |
| Olopatadine | Desloratadine | X | Moderate | Fair | 1 (0.3) |
| Diclofenac | Ibuprofen | X | Moderate | Fair | 5 (1.6) |
| Diphenhydramine | Salbutamol-Ipratropium | X | Moderate | Fair | 1 (0.3) |
| Salbutamol | Ipratropium | X | Moderate | Fair | 1 (0.3) |
| Fexofenadine | Ipratropium | X | Moderate | Fair | 1 (0.3) |
| Diclofenac | Ketorolac | X | Moderate | Fair | 2 (0.6) |
| Ibuprofen | Ketorolac | X | Moderate | Fair | 1 (0.3) |
| Ketotifen | Azelastine-Fluticasone | X | Moderate | Fair | 2 (0.6) |
| Ketotifen | Salbutamol-Ipratropium | X | Moderate | Fair | 1 (0.3) |
| Ketotifen | Olopatadine | X | Moderate | Fair | 1 (0.3) |
| Chlorpheniramine | Azelastine-Fluticasone | X | Moderate | Fair | 2 (0.6) |
| Category D | |||||
| Diphenhydramine | Hydroxyzine | D | Moderate | Fair | 6 (1.9) |
| Cetirizine | Hydroxyzine | D | Moderate | Fair | 3 (1) |
| Diphenhydramine | Chlorpheniramine | D | Moderate | Fair | 1 (0.3) |
| Methotrexate | Co-trimoxazole | D | Major | Good | 2 (0.6) |
| Fluticasone | Clarithromycin | D | Moderate | Excellent | 2 (0.6) |
| Category C | |||||
| Diphenhydramine | Chlorpheniramine | C | Moderate | Good | 14 (4.5) |
| Ketotifen | Chlorpheniramine | C | Moderate | Good | 12 (3.8) |
| Cetirizine | Diphenhydramine | C | Moderate | Good | 35 (11.3) |
| Cetirizine | Chlorpheniramine | C | Moderate | Good | 16 (5.1) |
| Ondansetron | Azithromycin | C | Moderate | Fair | 16 (5.1) |
Pharmacologic Details of the Drug-Drug Interactions Which Have the Risk Rating Interaction of X, D, and C a
| Drug 1 | Drug 2 | Risk Rating | Main Complication |
|---|---|---|---|
| Diclofenac | Ibuprofen | X | GI toxicity |
| Acetaminophen-Codeine | Chlorpheniramine | D | CNS suppression |
| Methotrexate | Cotrimoxazole | D | Pancytopenia |
| Dexamethasone | Ibuprofen | C | GI bleeding |
Interaction Risk Rating and Medical Complications of the Drugs with Major Interactions
Considering the severity of the identified DDIs, 93.6% of all interactions were moderate, while 6.4% were major. Additionally, more than half (64.5%) of the identified DDIs had excellent or good reliability constants, while 35.5% had moderate reliability constants. The prevalence of DDIs in patients who were visited by general physicians was 189 (26.6%), compared to 81 (26.9%) in patients who were visited by specialist or subspecialist physicians. No significant differences were observed regarding the type of physician (P = 0.938).
DDIs were more prevalent in patients with underlying conditions, with 37 (38.5%) of them reporting DDIs compared to 233 (25.5%) patients without underlying conditions. An underlying disease in patients increases the risk of DDIs, with an odds ratio of 1.8 (95% confidence interval = 1.2 - 2.8, P = 0.008).
5. Discussion
This study evaluated the prescription patterns of pediatric patients over nine months, focusing on DDIs and medication-related problems. The study found a significant occurrence of DDIs in pediatric patients in this population. The three most frequently observed interactions were between cetirizine and diphenhydramine, ondansetron and azithromycin, and cetirizine and chlorpheniramine, respectively. Furthermore, it was shown that the interaction between diclofenac and ibuprofen exhibited the highest level of severity. The DDI between two antihistamines was identified as the most prevalent. The administration of two antihistamines may not provide more efficacy and puts patients at an increased risk of adverse effects.
In our study, DDIs occurred at a high prevalence. A study by Jazber et al. on outpatient prescriptions revealed a lower DDI occurrence, with every 1 in 10 individuals exposed to clinically significant DDIs, whereas in our study about 1 in 4 patients were exposed (16). The most frequently observed DDI category in this study was C, which is consistent with the study conducted by Ren et al. (17).
Several factors could be associated with an increased susceptibility of a patient to experience DDIs. One of the key factors is the prevalence of an underlying disease. Patients with a past medical history tend to use medications more frequently, increasing the chance of DDIs. In our study, we found that the presence of an underlying disease significantly increases the chance of DDIs.
According to the findings of our study, antibiotics are the most frequently prescribed drugs by both general and specialist physicians, with azithromycin being the most frequently prescribed antibiotic. This high rate of antibiotic administration could lead to a high rate of drug-resistant organisms and also increase the chance of side effects and DDIs.
Similar findings were reported in a study on the pattern of drug consumption in outpatient departments in Iran by Aeenparast et al. The study highlighted the arbitrary consumption of common medicines by families, particularly those with lower incomes, less education, and older age, regardless of indication, side effects, and mechanisms of action. Delivering medicines that require a doctor's prescription without one, along with the increasing cost and wait time for a doctor's visit, has significantly influenced antibiotic use (18, 19).
In a study on the quality of prescription drugs used by outpatients in various Iranian cities, antibiotics were found to be the most commonly administered, followed by antihistamines, according to Naserifar et al. Ketotifen and diphenhydramine were the most common drugs in the antihistamine pharmacological class, particularly among children, which aligns with our research. Additionally, first-generation antihistamines have been utilized more frequently than second-generation antihistamines (20). Based on the safety profile of second-generation antihistamines in children, particularly in cases of overdose and fewer side effects, it could be beneficial for physicians to consider prescribing second-generation antihistamines (21).
Acetaminophen is widely used as an antipyretic and analgesic for outpatients, particularly in children and the elderly, and it is also commonly prescribed to the middle-aged population after ibuprofen. Despite the risk of liver toxicity, it is the most frequently prescribed drug for outpatients in Iran due to its low interaction profile, minimal adverse effects, and availability in multiple pharmaceutical forms at reasonable prices. Two separate studies by Almasi et al. and Mohammadi et al. validate the high prescribing prevalence of this drug in our study (22, 23).
Behnood-Rod et al. conducted a study on the reasons for outpatient pharmacy visits and found that herbal medicines are the second most common reason after vitamins. Upper respiratory tract diseases have led to an increase in the use of herbal medicines, either prescribed or self-administered (24).
A study by McPhillips et al., in the United States found that 15% of outpatient prescriptions for children had potential dosage errors. Analgesics were the most concerning, being more likely than other drugs to cause significant harm if overused. This study showed that only 67% of drugs are administered within the recommended dosage range for children weighing less than 35 kg, with over 1% being prescribed more than twice the maximum recommended dose (25).
Our study investigated one of the most prevalent medical and pharmaceutical issues in the healthcare setting, but it still has limitations. First, we had a small sample size in one center, which could affect our results. Additionally, cross-sectional studies cannot be generalized to represent the entire population of patients. Therefore, it is recommended to conduct a prospective cohort study and surveillance program. Potential issues such as selection bias, incomplete data, and the limitations of cross-sectional studies may compromise the generalizability of our findings.
In conclusion, DDIs occur at a high prevalence in the pediatric population in our center. Antihistamines are the drug class most frequently identified in DDIs. Underlying conditions put patients at an increased risk of experiencing DDIs. Physicians and pharmacists should be aware of the potential DDIs and at-risk populations. Additionally, antibiotics are frequently administered to patients, and this high rate of antibiotic use could lead to high rates of drug-resistant pathogens. Surveillance and auditing of prescriptions, especially in university-affiliated hospitals and clinics, are recommended to identify prescription patterns and DDIs, which could lead to decreased harm to patients and safer use of medications.