1. Background
Arterial stiffness (AS), a crucial indicator of cardiovascular disease (CVD), can strongly predict future vascular events and is closely linked to CVD risk factors and the development of atherosclerotic disease (1). Numerous studies have explored AS's capacity to predict future fatal and non-fatal CVD events (1, 2). Various methods, such as pulse wave velocity (PWV) measured through tonometry, oscillometry, or sonography, are used to assess AS. For instance, Urbina et al. used PWV to study AS changes in children with type I diabetes mellitus (DMTI) (2). Another method is the augmentation index (AIx) (3). However, a unique technique measures aortic strain parameters, namely aortic distensibility (AD), ASβ index (ASβI), and pressure strain elastic modulus (PSEM), using echocardiography with aortic diameters and blood pressures (4). Non-invasive evaluation of ASβI has been shown to be comparable to invasive methods, achieving a high degree of accuracy and leading to its widespread adoption and development. In this context, Noori et al. utilized aortic strain, ASβI, AD, and PSEM to assess changes in celiac disease (5), thalassemia (4), and obesity (1).
Arterial stiffness reflects the stiffness of arterial walls, with various factors influencing its degree of stiffening. Traditional CVD risk factors negatively impact arterial stiffness, with factors like hypertension, chronic kidney disease, and diabetes contributing to changes in arterial walls and increased stiffness (1). Additionally, age is a crucial factor in hypertension and can contribute to the progression of arterial stiffness. Studies have reported significantly higher hypertension and larger arteries in older individuals compared to younger ones. Diabetes, a significant contributor to CVD, increases the likelihood of arterial stiffening and cardiovascular disease, especially in children with type 1 diabetes mellitus (DMTI) (6). Abnormal chamber stiffening in polygenic diseases is complex and attributed to kidney impairment, metabolic disturbances, small vessel disease, cardiac autonomic dysfunction, and insulin resistance (7). In patients with diabetes mellitus (DM), AS is linked to insulin resistance, diabetes duration, and hemoglobin A1c (HbA1c) levels (1). Furthermore, ASβI and PSEM, as parameters of arterial stiffening, are significantly altered in patients with DM, with worse outcomes observed in those with prolonged diabetes duration and poor glycemic control (1, 8).
2. Objectives
Given the information above and the knowledge regarding elasticity status in diabetes, the present study aimed to evaluate differences in arterial stiffening between children with DMTI and healthy individuals, as well as to assess the effects of diabetes duration and HbA1c levels on these parameters.
3. Methods
3.1. Study Design
This case-control study involved 192 children, with 96 in each group of DMTI and healthy children aged 4 to 18. The study was conducted from January 2020 to June 2021 at the Cardiac Center of Ali Asghar Paediatric Hospital, affiliated with Zahedan University of Medical Sciences. Healthy children were selected from those visiting the pediatric clinic for heart examination and confirmed to be free of any diseases, especially heart diseases, through echocardiography. Symptomatic and asymptomatic children with DMTI were included in the study as patients with fasting blood sugar levels above 125 mg/dL and random blood sugar below 200 mg/dL to confirm the diabetes diagnosis. Exclusion criteria included age over 19 years and the presence of heart diseases such as systemic elevated blood pressure, ischemic heart disease, hypertrophic and dilated cardiomyopathy, valvular and congenital heart defects, and hypothyroidism. Abnormal BMI is a significant factor in stiffening, and to control the effect of obesity or underweight, these children were excluded from the study, as the aim was to assess the effect of diabetes.
3.2. Echocardiography Measures
Echocardiography was performed using a My Lab 60 machine with a three and eight transducer (manufactured in Italy). To ensure accuracy, measurements were repeated for three cycles, and the average was recorded. The diastolic and systolic diameters of the ascending aorta were assessed. These measurements were documented in M-mode from parasternal long-axis views, approximately 3 cm above the aortic valve.
3.3. Blood Pressure Measurement
Blood pressure (BP) measurements were taken in the supine position, with a minimum of 2-minute intervals between measurements, and the mean of three measurements was recorded. The first and fifth Korotkoff sounds were used to determine systolic and diastolic blood pressure, respectively.
3.4. Elasticity Parameters
The following formulas were used to calculate the elasticity parameters:
- Pressure Strain Elastic Modulus: (Systolic blood pressure - diastolic blood pressure)/[(systolic diameter - diastolic diameter)/diastolic diameter].
- Aortic Stiffness Beta Index: LN (systolic blood pressure * diastolic blood pressure/diastolic blood pressure)/((systolic diameter – diastolic diameter)/diastolic diameter).
- Arterial Strain (%): (Systolic diameter - diastolic diameter)/diastolic diameter × 100.
- Arterial Distensibility: 2 × (systolic diameter - diastolic diameter)/diastolic diameter/(systolic blood pressure - diastolic blood pressure).
3.5. Ethical Approval and Informed Consent
The study received ethical approval, and consent forms were signed by the participants or their guardians. The ethical code for the study is IR.ZAUMS.REC.1400.095.
3.6. Statistical Analysis
Data were entered into SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Normality was assessed to determine the data distribution type. The Mann-Whitney U test was used to compare the distributions. A P-value of ≤ 0.05 was considered statistically significant.
4. Results
The study included 192 children, evenly matched by age and gender, with 96 children in each group (DMTI and healthy controls). The mean age was 10.77 ± 2.82 years in the control group and 10.87 ± 3.46 years in the DMTI group. Boys constituted 47.9% of the cases and 57.3% of the controls. Most study variables displayed a non-normal distribution (P < 0.05) except for height (K.S = 0.074, P = 0.200), weight (K.S = 0.076, P = 0.200), aortic systolic diameter (K.S = 0.055, P = 0.200), and aortic diastolic diameter (K.S = 0.058, P = 0.200), which were normally distributed.
Table 1 presents the comparative analysis of study variables between children with DMTI and healthy children. All variables except for height (P = 0.204) and age (P = 0.579) were significantly different between controls and cases. BMI (P < 0.001), weight (P < 0.001), diastolic blood pressure (P < 0.001), systolic blood pressure (P = 0.002), systolic (P < 0.001), and diastolic (P = 0.005) aortic diameters were significantly higher in diabetic children. The stiffness parameters, including PSEM (P = 0.030) and ASβI (P < 0.001), were significantly higher in children with diabetes, whereas AD (P < 0.001) and AS (P < 0.001) were higher in healthy children.
| Variables and Groups | Values a | MW | P-Value |
|---|---|---|---|
| Age | 4395 | 0.579 | |
| Case | 10.87 ± 3.46 | ||
| Control | 10.77 ± 2.82 | ||
| Height | 4119 | 0.204 | |
| Case | 137.39 ± 19.01 | ||
| Control | 141.82 ± 14.01 | ||
| Weight | 2998 | < 0.001 | |
| Case | 33.29 ± 11.94 | ||
| Control | 39.84 ± 9.55 | ||
| BMI | 3160.5 | < 0.001 | |
| Case | 16.1 ± 2.55 | ||
| Control | 16.84 ± 3.01 | ||
| SBP | 3456 | 0.002 | |
| Case | 101.71 ± 8.57 | ||
| Control | 98.4 ± 10.17 | ||
| DBP | 3100 | < 0.001 | |
| Case | 66.6 ± 7.26 | ||
| Control | 61.84 ± 9.28 | ||
| AOS | 2821 | < 0.001 | |
| Case | 21.89 ± 2.94 | ||
| Control | 19.9 ± 2.97 | ||
| AOD | 3524 | 0.005 | |
| Case | 19.65 ± 2.93 | ||
| Control | 18.55 ± 3.11 | ||
| ASβI | 2915.5 | < 0.001 | |
| Case | 12.71 ± 15.15 | ||
| Control | 8.77 ± 17.46 | ||
| AS | 3149 | < 0.001 | |
| Case | 7.78 ± 6.32 | ||
| Control | 11.98 ± 9.1 | ||
| AD | 2792.5 | < 0.001 | |
| Case | 0.08 ± 0.06 | ||
| Control | 0.13 ± 0.09 | ||
| PSEM | 3774.5 | 0.03 | |
| Case | 8.92 ± 12.03 | ||
| Control | 7.24 ± 14.34 |
Abbreviations: SD, standard deviation; MW, Mann-Whitney U test; BMI, Body Mass Index; SBP, systolic blood pressure; DBP, diastolic blood pressure; AOS, systolic diameter of the aorta; AOD, diastolic diameter of the aorta; AS, aortic strain; AD, aortic distensibility; PSEM, pressure strain elastic modulus; ASβI, aortic stiffness β index.
a Values are expressed as mean ± SD.
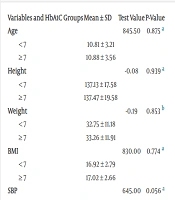
Table 2 shows the results of changes in study variables and stiffening parameters based on HbA1c levels, categorized into good control (< 7) and poor control (≥ 7). DBP (P = 0.025) and diabetes duration (P = 0.002) were significantly different. All stiffness parameters were higher in diabetic children with good control but were not statistically significant.
| Variables and HbA1C Groups | Mean ± SD | Test Value | P-Value |
|---|---|---|---|
| Age | 845.50 | 0.875 a | |
| < 7 | 10.81 ± 3.21 | ||
| ≥ 7 | 10.88 ± 3.56 | ||
| Height | -0.08 | 0.939 a | |
| < 7 | 137.13 ± 17.58 | ||
| ≥ 7 | 137.47 ± 19.58 | ||
| Weight | -0.19 | 0.853 b | |
| < 7 | 32.75 ± 11.18 | ||
| ≥ 7 | 33.26 ± 11.91 | ||
| BMI | 830.00 | 0.774 a | |
| < 7 | 16.92 ± 2.79 | ||
| ≥ 7 | 17.02 ± 2.66 | ||
| SBP | 645.00 | 0.056 a | |
| < 7 | 99.00 ± 7.56 | ||
| ≥ 7 | 102.61 ± 8.74 | ||
| DBP | 611.00 | 0.025 a | |
| < 7 | 63.54 ± 6.16 | ||
| ≥ 7 | 67.63 ± 7.35 | ||
| AOS | 0.21 | 0.835 b | |
| < 7 | 22.00 ± 2.93 | ||
| ≥ 7 | 21.85 ± 2.97 | ||
| AOD | 0.46 | 0.574 b | |
| <7 | 19.95 ± 2.71 | ||
| ≥ 7 | 19.55 ± 3.02 | ||
| ASβI | 854.00 | 0.933 a | |
| <7 | 16.10 ± 22.29 | ||
| ≥ 7 | 11.59 ± 11.88 | ||
| AS | 860.00 | 0.973 a | |
| < 7 | 9.16 ± 8.97 | ||
| ≥ 7 | 7.33 ± 5.14 | ||
| AD | 862.50 | 0.990 a | |
| < 7 | 0.08 ± 0.07 | ||
| ≥ 7 | 0.08 ± 0.05 | ||
| PSEM | 817.00 | 0.691 a | |
| < 7 | 11.94 ± 18.90 | ||
| ≥ 7 | 7.92 ± 8.60 | ||
| Duration | 492.50 | 0.002 a | |
| < 7 | 43.88 ± 21.66 | ||
| ≥ 7 | 27.67 ± 22.78 |
Abbreviations: SD, standard deviation; BMI, Body Mass Index; SBP, systolic blood pressure; DBP, diastolic blood pressure; AOS, systolic diameter of the aorta; AOD, diastolic diameter of the aorta; AS, aortic strain; AD, aortic distensibility; PSEM, pressure strain elastic modulus; ASβI, aortic stiffness β index.
a Mann-Whitney U test.
b Independent t-test.
Table 3 presents the results of the comparative analysis of study variables and stiffness parameters in two groups of diabetic children categorized based on the duration of diabetes, with a cutoff of 24 months. All variables differed between these two groups; however, statistically significant differences were observed only for AD (P = 0.042) and HbA1c (P = 0.033).
| Variables and Diabetes Duration | Mean ± SD | Test Value | P-Value |
|---|---|---|---|
| Age | 957 | 0.167 a | |
| ≤ 24 | 10.37 ± 3.60 | ||
| > 24 | 11.46 ± 3.23 | ||
| Height | -1.88 | 0.063 b | |
| ≤ 24 | 134.08 ± 19.97 | ||
| > 24 | 141.30 ± 17.22 | ||
| Weight | -1.45 | 0.151 b | |
| ≤ 24 | 31.56 ± 12.39 | ||
| > 24 | 35.00 ± 10.60 | ||
| BMI | 1088.5 | 0.683 a | |
| ≤ 24 | 16.85 ± 2.69 | ||
| > 24 | 17.17 ± 2.68 | ||
| SBP | 1133 | 0.934 a | |
| ≤ 24 | 102.08 ± 8.76 | ||
| > 24 | 101.27 ± 8.41 | ||
| DBP | 1062 | 0.529 a | |
| ≤ 24 | 67.10 ± 7.14 | ||
| > 24 | 66.02 ± 7.44 | ||
| AOS | 0.04 | 0.968 b | |
| ≤ 24 | 21.90 ± 3.17 | ||
| > 24 | 21.88 ± 2.69 | ||
| AOD | -0.3 | 0.765 b | |
| ≤ 24 | 19.57 ± 3.25 | ||
| > 24 | 19.75 ± 2.55 | ||
| ASβI | 902 | 0.075 a | |
| ≤ 24 | 14.49 ± 15.80 | ||
| > 24 | 10.62 ± 14.25 | ||
| AS | 926 | 0.109 a | |
| ≤ 24 | 6.76 ± 5.58 | ||
| > 24 | 8.99 ± 6.96 | ||
| AD | 868 | 0.042 a | |
| ≤ 24 | 0.06 ± 0.05 | ||
| > 24 | 0.09 ± 0.07 | ||
| PSEM | 962.5 | 0.182 a | |
| ≤ 24 | 9.70 ± 11.61 | ||
| > 24 | 8.00 ± 12.58 | ||
| HbA1c | 855 | 0.033 a | |
| ≤ 24 | 8.95 ± 2.08 | ||
| > 24 | 8.10 ± 1.65 |
Abbreviations: SD, standard deviation; BMI, Body Mass Index; SBP, systolic blood pressure; DBP, diastolic blood pressure; AOS, systolic diameter of the aorta; AOD, diastolic diameter of the aorta; AS, aortic strain; AD, aortic distensibility; PSEM, pressure strain elastic modulus; ASβI, aortic stiffness β index; HB A1c, hemoglobin A1c.
a Mann-Whitney U test.
b Independent t-test.
5. Discussion
Atherosclerotic damage in children arises from structural collapse, intima repair, thickening, and arterial wall deformity, increasing the risk of sudden cardiac arrest, often undiagnosed until adolescence (1, 4, 5). In most cases, arterial changes are mild and can go unnoticed, with lifestyle improvements offering some mitigation. However, children with DMTI face a significantly elevated risk, up to 10 times, of developing atherosclerosis and experiencing heart damage compared to the general population (9). Our study revealed higher PSEM and ASβI, and lower AD and AS in diabetic children. Interestingly, these parameters did not show significant changes based on HbA1c levels, but AD varied with the duration of diabetes.
Previous studies in this domain have predominantly focused on using PWV and AIx as stiffness parameters, with limited research exploring aortic strain, ASβI, AD, and PSEM (1, 4, 5). Mamo et al. (8) and Obermannova et al. (9) demonstrated the association of diabetes and impaired glucose tolerance with AS changes. Hence, achieving better glycemic control in children with DMTI is vital to prevent damage to AS parameters.
Additionally, Galler et al. reported significantly lower AD in children with DMTI than in healthy children (10). McCulloch et al. conducted an MRI study to assess AS in children with DMT1 (11). They found no significant decrease in strain and distensibility compared to controls, while Çiftel et al. reported a reduction in aortic strain and aortic distensibility in the diabetic group (12). Moreover, Zoppini et al. evidenced a significant increase in ASβI in patients with DMTI (13). Agnoletti et al. also reported minimal alterations in AS parameters among diabetic patients, except in the presence of arterial hypertension (14).
Various studies have measured different features with different intentions in this area. Liang et al. observed a significant connection between HbA1c levels and an increase in PWV, even after controlling for age, sex, and BMI. Furthermore, this association remained significant even after considering additional factors such as blood pressure, heart rate, and lipids (15). Kozakova et al. indicated that HbA1c had an independent relationship with PWV, with higher PWV observed in individuals with HbA1c ≥ 6.5% (16). There was also a connection between diabetes duration and AS, as discovered by Tentolouris et al. (17). Liu et al. acknowledged that patients receiving insulin showed higher AS (18), while Rosenlund et al. found that patients receiving continuous subcutaneous insulin infusion displayed lower AS compared with those treated by multiple insulin injections (19). These findings suggest that normalizing glucose levels with constant insulin infusion may protect vascular walls and counteract their stiffening. However, in the present study, the structural factors of the heart, namely PSEM, ASβI, AD, and AS, did not change in the groups of diabetic children based on the level of HbA1c. This difference from the results of the above-mentioned studies is probably due to different parameters of heart stiffening.
Urbina et al. showed higher PWV in children with DMTI after adjusting for age (2). Blood pressure levels have been closely associated with AS, with every 2.1 mmHg elevation of systolic blood pressure or 1.7 mmHg elevation of pulse pressure resulting in a 1 m/s rise of PWV in children with DMTI. Wadwa et al. compared AS parameters in DMTI and DMTII (type 2 diabetes mellitus) patients and found that those with DMTII had worse PWV (20). This may be attributed to increased central obesity and blood pressure in DMTII, as both are connected to arterial stiffness regardless of diabetes type. The mentioned results suggest that children with DMTII are at a higher risk of future cardiac complications (20).
Castro-Correia et al. declared that most diabetic children had high PWV, even when there were no significant differences in patients with poor glycemic control compared to those with reasonable diabetes control (21). They also observed a significant correlation between AS and the duration of diabetes, indicating that even very young children with minimal disease advancement time are susceptible to an increase in stiffness. Terlemez et al. aimed to identify atherosclerosis risk in DMTI children without vital organ damage or CVD and found significantly higher PWV and AIx values in DMTI children compared to controls. They also found a correlation between the polygenic disorder period, HbA1c levels regarding the PWV, and AIx values (22).
Chang et al. found that short-term glycemic control had no effects on blood vessel stiffness in diabetic patients. However, they reported that changes in HbA1c levels were connected to early vascular changes in DMTI (23). The impact of these metabolic disturbances on myocardial structure and performance is yet to be fully understood (24).
Regarding the above-mentioned data, since adolescence is a crucial phase connected to the emergence and progression of vascular complications, it is substantial to assess the peripheral and central cardiac vascular alterations. In our study, the presence of diabetes mellitus in children showed changes in AS, but poor diabetes control did not result in any changes in the patients. However, diabetes duration significantly affected AD in these patients, which is consistent with the findings of previous studies.
It is essential to acknowledge the limitations of our study. We did not follow patients prospectively to predict heart events, so we were not able to declare if AS determines cardiac diseases. Additionally, the cross-sectional nature of the study limits our ability to determine if these fluctuations are stable or only emerge in the acute phase of the disease.
In conclusion, we found that children with DMTI exhibited higher stiffness parameters, such as ASβI and PSEM, and significantly lower AS and AD values than healthy children. However, the type of diabetes control and the disease duration did not significantly impact the stiffening parameters, except for AD, which was influenced by the duration of diabetes. These findings highlight the importance of monitoring aortic elasticity parameters in children with diabetes, particularly those with a longer disease duration. By closely monitoring and maintaining better control of diabetes, healthcare professionals can potentially mitigate the risk of future cardiovascular events in this population. Further research is warranted to check out the long-term implications of AS in children with diabetes and to develop targeted interventions to improve cardiovascular outcomes in this vulnerable population.