1. Background
At the end of December 2019, a new coronavirus called COVID-19 caused the outbreak of pneumonia from Wuhan to the whole world (1). In Wang et al. study, the estimated death rate due to coronavirus was reported as 2.84% based on the total number of patients (2, 3). The initial symptoms of this disease include pneumonia, fever, myalgia, and fatigue. It is difficult to distinguish patients with COVID-19 from healthy individuals. In the cases of confirmed COVID-19, it has been reported that the clinical symptoms are not unique to COVID-19, as these symptoms are like those of other viral diseases such as influenza and colds (4). Nevertheless, studies in this field have already started and will continue both inside our country and worldwide, yet the prevention and control of infection and observance of health principles by the general population remain the main priority (5). Since the onset of COVID-19, numerous studies have documented prolonged symptoms following recovery, commonly referred to as prolonged COVID-19 or the post-COVID-19 state (6). As per the World Health Organization (WHO) definition, post-COVID-19 condition in children encompasses a history of confirmed SARS-CoV-2 infection, accompanied by at least one persistent physical symptom lasting for a minimum of 12 weeks following the initial testing. Symptoms that persist after a COVID-19 infection may impact daily activities and may fluctuate or reoccur over time (7). These symptoms encompass shortness of breath, chronic cough, chest tightness, as well as cognitive impairment, and severe fatigue (8, 9). Monitoring studies of post-COVID-19 pulmonary function tests (PFTs) in adults have shown altered respiratory function, with abnormal diffusing capacity being the most common finding, appearing in 40% to more than 50% of patients in most studies (10-12). In a percentage of children with COVID-19, there is pulmonary involvement, and this pulmonary involvement needs time to recover, and even some experts, based on evidence-based studies in adults, suggest that this pulmonary involvement can last for a long time after recovery. Viral infection can also cause patterns of pulmonary function impairment in PFTs (13). In the studies conducted on patients affected with influenza, it has been seen that despite the complete improvement in clinical symptoms, spirometry parameters remain disturbed for a long time (about 6 months), which can be caused by underlying hyperactive diseases. Most of the patients who recovered from COVID-19 have an irritating cough that can be accompanied by spirometry involvement. Determining these disorders can indicate the simultaneous presence of an underlying disease (asthma, underlying hyperactive diseases) and lead to their long-term follow-up and treatment (11, 13, 14). Spirometry is still considered the most common and at the same time the most popular lung function test among adults and cooperative children older than 6 years, which does not require sedation and can be performed with simple training, and its correct interpretation is also very helpful in the diagnosis (14). So far, no study has been published regarding spirometry findings in children who have recovered from COVID-19, while in studies of adults, significant changes in the phase of recovery from COVID-19 have been reported;
2. Objectives
Therefore, we decided to perform spirometry in children recovered from COVID-19 in the recovery phase to investigate the frequency of abnormal findings and its relationship with the severity of primary lung involvement and inflammatory markers.
3. Methods
This study is a cross-sectional one. In the PCR-positive patients, all children between the ages of 6 and 14 from March 2021 to March 2023 who were admitted to the COVID-19 department of Akbar referral Hospital, in whom the diagnosis of COVID-19 was confirmed with either a positive nasopharyngeal PCR test for SARS-CoV-2 or a positive IgM antibody serology against SARS-CoV-2 in addition to a CT scan showing evidence of pulmonary involvement, were included. Among the PCR-negative patients, all children in the age range of 6 to 14 years admitted to Akbar hospital's COVID department, with a negative nasopharyngeal PCR test result for SARS-CoV-2 but showing evidence of pulmonary involvement in their CT scan and whom their parents consented to participate in the study were also recruited. The inclusion criteria for this study required children aged 6 to 14 years hospitalized in the COVID ward of Akbar hospital. For the PCR-positive group, patients needed a positive nasopharyngeal PCR test for SARS-CoV-2 or positive IgM antibody serology for SARS-CoV-2, with lung involvement visible on CT scans. The PCR-negative group included children with negative nasopharyngeal PCR tests or negative IgM serology for SARS-CoV-2 who have lung involvement on CT scans at the same time. Exclusion criteria included a history of lung disease affecting lung function (such as asthma even mild type, bronchopulmonary dysplasia, prematurity, or cystic fibrosis), parental unwillingness to continue participation, or lack of cooperation in completing spirometry for the second 3-month post-recovery period. Additionally, children unable to perform spirometry and those with contraindications for spirometry (such as chest pain, pneumothorax, recent eye, abdominal, or chest surgery, and active respiratory infections, including tuberculosis, cold, flu, or acute COVID-19) were excluded. The patients were subjected to spirometry twice in two different periods (the first 3 months and the second 3 months after recovery from COVID-19) and the relationship between changes in spirometry parameters with the severity of lung involvement based on CT scan and the increase in inflammatory markers including CRP were measured. It should be noted that the spirometry costs were paid from the researcher's grant and were not covered by the patient's insurance. Regarding the spirometry test, it should be noted that the forced expiratory volume (FEV) 1 and forced vital capacity (FVC) indices and the FEV1 to FVC ratio were measured by the CHEST HI-105 spirometry device in children. In the spirometry test, a tube is inserted into the person's mouth, and they must breathe in it. This tube is connected to a small computerized device. A technician directs the test and instructs the person to take a deep, full breath and then exhale quickly and forcefully. This should be done several times. In the spirometry device, the amount of exhaled air and its speed are displayed. This measurement provides accurate information about a person's lung function. With full breathing and strong exhalation in the spirometer, the following parameters are measured: (1) Forced vital capacity: This is the volume of air that a person exhales forcefully. This measurement is different from the vital capacity, which is the maximum normal exhaled air; (2) active expiratory volume in the first second (FEV1): This is the volume of air that a person exhales forcefully in one second; and (3) the FEV1 to FVC ratio or FEV1/FVC. Contraindications for using spirometry include chest pain, pneumothorax, recent eye, abdominal, or chest surgery, and active respiratory infections such as tuberculosis, influenza, flu, or COVID-19 in the acute phase. This study examined several variables across demographic, clinical, pulmonary function, radiological, and time-dependent categories. Demographic variables included age and sex distribution. Group assignment was based on PCR test results (positive or negative for COVID-19). Clinical measures incorporated CRP levels. Pulmonary function test variables included FEV1, FVC, FEV1/FVC ratio, and FEF25.75, measured as percentages. Radiological findings on CT scans included ground-glass opacities (GGO), pleural thickening, interlobular septal thickening, air bronchogram, and consolidation, as well as various combinations of these findings, such as GGO with pleural thickening or consolidation. In the PCR-positive group, spirometry values (FEV1, FVC, FEV1/FVC ratio, and FEF25.75) were measured at two time points (first and second 3 months after COVID-19 recovery) to assess time-dependent changes. Additionally, the study explored associations between specific CT findings and changes in spirometry outcomes over time, including significant relationships between interlobular septal thickening and both FVC and FEV1 percentages, as well as between GGO, GGO consolidation, and pleural thickening with FEV1/FVC and FEF25.75 percentages in the second 3 months post-recovery. This research has been approved by the ethics committee of Mashhad University of Medical Sciences, IR.MUMS.MEDICAL.REC.1400.764. Eventually, the collected data were analyzed using descriptive and inferential statistics by the SPSS software ver. 21. The variables were reported as frequency, percentage, mean, and standard deviation. Comparison between the two groups was done with an independent t-test. The relationship between the qualitative variables was investigated with a chi-square test. In all analyses, the significance level was considered as a P < 0.05.
4. Results
In this research, 50 patients, 25 in the PCR-positive and another 25 in the PCR-negative group, were examined. Both groups consisted of 15 (60%) males and 10 (40%) females. The mean age of the patients was 9.00 ± 2.35 years (minimum 6 and maximum 14 years). The mean age of the children in the PCR-negative group was 7.88 ± 1.53 years, whereas it was 10.12 ± 2.52 years in the PCR-positive group. t-test results showed a statistically significant difference between the two groups in terms of age (P = 0.01). However, no significant difference was achieved between the mean FEV1 (P = 0.5), FVC (P = 0.4), and FEV1/FVC ratio (P = 0.4) of the two groups. The mean FEF25.75 (P = 0.01) and CRP (P = 0.01) of the patients in the two studied groups showed a statistically significant difference as the mean FEF25.75 in the PCR-negative group and the mean CRP in the PCR-positive group was significantly higher (Table 1). In addition, in the PCR-positive group, the patients underwent spirometry once again in the second 3 months after recovery from COVID-19 to investigate the relationship between changes in spirometry parameters and the severity of lung involvement. The results of the paired t-test showed the mean FEV1 (P = 0.01), FVC (P = 0.01), and FEV1/FVC ratio (P = 0.01) of the patients in the second 3 months after recovery from COVID-19 was significantly reduced compared to the first 3 months after recovery (Table 2).
| PCR | Mean ± SD | P-Value | |
|---|---|---|---|
| Negative | Positive | ||
| FEV1 (%) | 93.08 ± 20.49 | 89.86 ± 17.44 | 0.5 |
| FVC (%) | 98.41 ± 11.68 | 95.28 ± 17.73 | 0.4 |
| FEV.1/FVC (ratio/%) | 99.88 ± 10.45 | 97.62 ± 8.92 | 0.4 |
| FEF25.75 (%) | 92.67 ± 23.77 | 59.09 ± 29.66 | 0.01 |
| CRP | 17.72 ± 6.92 | 46.92 ± 28.82 | 0.01 |
Evaluation and Determination of the Other Investigated Variables in the Studied Groups (PCR-Positive and Negative)
| PCR-Positive | Mean ± SD | Positive PCR (P-Value) | |
|---|---|---|---|
| The First 3 Months After Recovery | The Second 3 Months After Recovery | ||
| FEV1 (%) | 89.86 ± 17.44 | 81.96 ± 16.18 | 0.01 |
| FVC (%) | 95.28 ± 17.73 | 89.00 ± 16.40 | 0.01 |
| FEV1.FVC (ratio/%) | 97.62 ± 8.92 | 91.84 ± 8.53 | 0.01 |
Investigating the Changes in Spirometry Parameters During the Recovery Phase from COVID-19
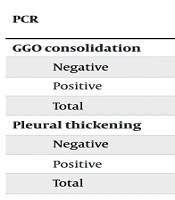
When examining the CT scan findings of the studied patients, GGO was diagnosed in 7 (14%), pleural thickening in 2 (4%), interlobular septal thickening in 3 (6%), air bronchogram in 2 (4%), GGO + Interlobular septal thickening in 8 (16%), GGO + pleural thickening in 7 (14%), GGO + air bronchogram in 2 (4%), GGO + pleural thickening + Interlobular septal thickening in 1 (2%), GGO + consolidation + pleural thickening in 10 (20%), GGO + consolidation + pleural thickening + interlobular septal thickening in 1 (2%), GGO + consolidation + interlobular septal thickening in 2 (4%), GGO + pleural thickening + air bronchogram in 2 (4%), and GGO + consolidation + air bronchogram was observed in 1 (2%) of the studied cases. Chi-square test results showed that CT scan findings including GGO consolidation (P = 0.01) and pleural thickening (P = 0.02) were significantly higher in the PCR-positive group (Table 3). However, there was no statistically significant difference in the findings of interlobular septal thickening (P = 0.7) and air bronchogram (P = 0.4) on CT scans between the two studied groups. The t-test results showed that the mean percentage of FVC in the first 3 months after recovery from COVID-19 was significantly higher among those who had interlobular septal thickening on CT scan (P = 0.009). Moreover, the mean percentage of FEV1 in the second 3 months after recovery from COVID-19 was significantly higher among those who had interlobular septal thickening on CT scan (P = 0.03). The mean percentage of FEV1/FVC ratio in the second 3 months after recovery from COVID-19 among those who had GGO on CT scan findings was significantly lower (P = 0.008), while it was significantly higher among those who had GGO consolidation in CT scan findings (P = 0.008). Moreover, the mean percentage of FEF25.75 in the second 3 months after recovery from COVID-19 was significantly lower among those who had evidence of GGO (P = 0.01) and pleural thickening (P = 0.04) on CT scan, whereas it was significantly higher among those who had GGO consolidation (P = 0.01).
| PCR | No. (%) | P-Value | |
|---|---|---|---|
| Yes | No | ||
| GGO consolidation | 0.01 | ||
| Negative | 2 (8.0) | 23 (92.0) | |
| Positive | 15 (60.0) | 10 (40.03) | |
| Total | 17 (34.0) | 33 (66.0) | |
| Pleural thickening | 0.02 | ||
| Negative | 8 (32.0) | 17 (68.0) | |
| Positive | 16 (64.0) | 9 (36.0) | |
| Total | 24 (48.0) | 26 (52.0) | |
Investigating the Frequency of Ground-Glass Opacities Consolidation and Pleural Thickening in the Studied Groups
5. Discussion
This study found that in the first 3 months post-recovery, PCR-negative patients exhibited a significantly higher mean FEF25.75, while PCR-positive patients showed a higher mean CRP, suggesting lingering inflammation in the latter group. In the PCR-positive group, spirometry in the second three months revealed significant reductions in FEV1, FVC, and the FEV1/FVC ratio compared to the first three months, indicating a decline in lung function over time. Additionally, CT scans showed that GGO consolidation and pleural thickening were notably more common in PCR-positive patients. The elevated CRP and persistent imaging findings suggest that COVID-19 may have prolonged effects on lung health, potentially affecting peripheral airways even in initially mild cases. These findings are consistent with other studies, including one involving 34 children, where 50% of clinically followed patients exhibited pulmonary complications one-month post-infection, indicating prolonged lung effects (15). Similarly, Moreno-Pérez et al. defined post-COVID syndrome (PCS) as persisting clinical symptoms or lung abnormalities on spirometry or radiography, with 50% of 270 adult patients displaying PCS and 25% showing abnormal spirometry findings post-recovery (14). Torres-Castro et al. reported, through a meta-analysis of 380 COVID-19 patients, a prevalence of reduced carbon monoxide diffusing capacity, restrictive lung pattern, and obstructive lung pattern at rates of 0.39, 0.15, and 0.07, respectively, post-recovery (11). Additionally, a case series by Fumagalli et al. documented decreased FEV1 and FVC levels in COVID-19 recovery phases, with some improvement over 6 weeks but sustained below-normal FVC (13). Persistent reductions in pulmonary function post-COVID-19 have been similarly observed, including reduced CO diffusing capacity and prolonged recovery in spirometric values, especially FVC, even after moderate to severe cases of COVID-19 (16-19). While there is limited data on mild cases, a study observed that symptomatic individuals showed reduced FEV1, vital capacity, and CO2 diffusing capacity 2 months post-infection (20). By contrast, studies in children, such as those conducted in Switzerland and by Vezir et al., revealed no spirometric alterations in asymptomatic or mild cases several months post-infection, suggesting severity may influence the risk of persistent effects (21, 22). In pediatric cases, several studies, including those by Bottino et al. and Vezir et al., reported no lung abnormalities in spirometry, airway resistance, or DLCO among asymptomatic or mildly symptomatic patients post-COVID-19 (22, 23). This highlights that while mild cases may show a favorable recovery, PCR-positive cases with increased CRP levels and CT abnormalities like GGO consolidation and pleural thickening may have more sustained respiratory effects. Our findings corroborate the hypothesis that COVID-19 may impose prolonged respiratory impacts in children, even in cases with mild initial symptoms, emphasizing the need for further investigation and monitoring of pediatric lung health following COVID-19. This study contributes valuable data for understanding the long-term implications of COVID-19 on children’s respiratory health and offers insight for future diagnostic, preventive, and therapeutic strategies. The strengths of this study include its novel concept and methodology, as there are very few similar studies in this field. However, one limitation is that it was conducted in a single center, which may reduce the generalizability of the findings. To address this, future research should focus on confirming these results through additional studies. Moreover, further investigations into the factors influencing the observed outcomes would provide valuable insights for future studies. Additionally, to enhance the generalizability of the findings, larger sample sizes and multi-center studies should be conducted. It is noteworthy that the absence of confidence intervals or confounder adjustments in the reported results limits the ability to generalize or assess the precision and validity of the findings.
5.1. Conclusions
Our study reveals significant and lasting respiratory effects in children recovering from COVID-19, with elevated CRP levels and a higher prevalence of GGO consolidation and pleural thickening in PCR-positive cases. These findings indicate persistent inflammatory responses and potential lung involvement, even in children without severe initial symptoms, highlighting the prolonged impact of COVID-19 on pediatric respiratory health.