1. Context
Hypercalcemia in pediatric patients, while less common than in adults, remains a critical aspect of diagnosis and treatment. Although there are similarities in the differential diagnosis between children and adults, notable differences exist in the etiological and epidemiological factors. Congenital causes are more common in children, contrasting with the predominance of acquired causes such as malignancy in adults. Additionally, the age of the child plays a crucial role, with neonates frequently affected by congenital anomalies, while adolescents exhibit conditions more typically observed in adults (1).
Hypercalcemia includes a wide spectrum of differential diagnoses, with primary hyperparathyroidism (PHPT) and malignancy accounting for approximately 80 to 90% of all cases. Among ambulatory patients, PHPT is more common, whereas malignancy is the leading cause, comprising up to 65% of cases in clinically ill and hospitalized patients (2).
2. Objectives
This article provides a concise overview of calcium metabolism and hypercalcemia in pediatric patients.
3. Evidence Acquisition
To achieve the goals of this study, a literature search was performed for English articles using keywords: Calcium, Calcium metabolism disorders, Hypercalcemia, Parathyroid hormone, Vitamin D, and Acid-Base imbalance. The search covered PubMed, Scopus, Web of Sciences, Cochrane, and Embase databases from 2000 to 2024.
This study provides a comprehensive review of important issues related to calcium metabolism and hypercalcemia:
(a) Overview of Calcium Metabolism
(b) Factors affecting serum total and ionized calcium concentration
(c) Clinical manifestations and importance of Hypercalcemia in pediatric patients
(d) Overview of hypercalcemia causes in pediatric patients
(e) Overview of hypercalcemia management
4. Results
4.1. Overview of Calcium Metabolism
Calcium is the most abundant mineral in the human body, predominantly an extracellular electrolyte, essential for regulating cell function. Ninety-nine percent of calcium is stored in the bones, and the remainder in the extracellular fluid (ECF) is divided as follows: 40 - 45% bound to albumin, 10 - 15% anion-bound (to anions like phosphate, lactate, and citrate, etc.), and 40 - 45% as the active ionized calcium (Ca2+), which is crucial in regulating cell action potential (3).
When calcium levels fluctuate in the bloodstream, a G protein-coupled receptor called the calcium-sensing receptor (CaSR) detects these changes in two primary sites: The parathyroid glands and the kidneys. The function of this receptor is crucial in bone and mineral metabolism. As calcium levels decrease in the ECF, CaSR signaling initiates two main responses: First in the parathyroid glands to release PTH and second in the kidneys, increasing the resorption of calcium; thus, raising serum calcium concentration (4).
The significance of the CaSR for these processes becomes evident when a loss or gain of function mutation occurs, causing disorders like familial hypocalciuric hypercalcemia (FHH) and autosomal dominant hypocalcemia (5).
PTH facilitates bone resorption by activating osteoclasts. It also increases the resorption of calcium and the excretion of phosphorus (via an increase in the production of Fibroblast Growth Factor-23 or FGF-23) and facilitates the production of 1,25-Dihydroxyvitamin D3 (1,25(OH)2 D3) in the kidneys (6, 7).
1,25(OH)2 D3 regulates intestinal calcium absorption by activating the intracellular vitamin D receptor (VDR) to stimulate gene expression, which increases the synthesis of calbindin-C, a transporter that facilitates the active transport of calcium through the intestinal cells. 1,25(OH)2 D3 also facilitates the absorption of phosphorus in the intestines. It is also important to note that active vitamin D increases the reabsorption of calcium and phosphorus in renal tubules, making it a vital regulator of these two electrolytes (8, 9).
4.2. Factors Affecting Serum Calcium Concentration
Every 1 mg/dL of calcium is equivalent to 0.25 mmol/L and 0.5 mEq/L. Thus, the normal range of total serum calcium is considered to be 8.8 to 10.3 mg/dL (equivalent to 2.2 to 2.6 mmol/L or 4.4 to 5.2 mEq/L). A calcium level above 10.5 mg/dL is defined as hypercalcemia and is categorized as mild (10.5 to 11.9 mg/dL), moderate (12.0 to 13.9 mg/dL), and severe or hypercalcemic crisis (14.0 to 16.0 mg/dL). Variations in albumin levels and hydration status affect serum calcium, explaining the broad range for normal total serum calcium; therefore, relying solely on measuring total calcium can be misleading since this parameter may change without an actual shift in ionized calcium levels. Similarly, the ionized portion can change without a variation in total calcium (5).
Conditions that alter the amount of serum protein can affect the total calcium concentration without necessarily impacting the ionized portion, a situation known as pseudohypocalcemia and pseudohypercalcemia. These conditions include diseases where serum protein content is increased (like multiple myeloma, etc.) or decreased (like cirrhosis, nephrotic syndrome, etc.). Prolonged venous occlusion (using a tight tourniquet) may also cause "ultrafiltration" of blood in the capillaries, thus artificially increasing the serum protein content of the blood sample and consequently causing an overestimation of calcium levels. One commonly used formula in clinical practice to estimate total calcium compensates for hypoalbuminemia by adding 0.8 mg/dL to the laboratory-measured calcium concentration for every 1.0 g/dL decrease in serum albumin concentration (10):
Although this formula has been widely used, its accuracy is questioned in more contemporary studies, especially in special populations, including critically ill patients, infants under one year of age, and patients with advanced chronic kidney disease (CKD). Consequently, measuring ionized calcium remains the gold standard for assessing calcium status, given the potential inaccuracies of calcium correction formulas (11).
Additionally, the total calcium concentration can be normal while the ionized fraction is altered, commonly observed in acid-base disorders. An increase in extracellular pH (alkalemia) enhances calcium binding to albumin, resulting in a decreased ionized calcium concentration. For instance, in acute respiratory alkalosis, each 0.1 unit increase in pH from the normal pH of 7.4 causes a 0.16 mg/dL decrease in ionized calcium concentrations. Therefore, various causes of acute respiratory alkalosis, such as hyperventilation, can provoke symptoms of hypocalcemia, including seizures, paresthesia, and muscle cramps (12).
4.3. Clinical Manifestations and Importance of Hypercalcemia in Pediatric Patients
Clinical manifestations of hypercalcemia, regardless of etiology or age of onset, include fatigue, muscle weakness, abdominal pain, anorexia, polydipsia, nausea, vomiting, headache, constipation, weight loss, polyuria, and fever. Chronic hypercalcemia can lead to nephrocalcinosis, which may decrease renal function. It can also cause renal colic and hematuria due to nephrolithiasis. Bone involvement may result in back pain, gait disturbances, lower limb deformities, fractures, limb bone tumors, and compression fractures of the vertebrae. Severe abdominal pain may occur and can be linked to acute pancreatitis or be mistaken for an acute abdomen. Hypercalcemic crisis is characterized by serum calcium levels exceeding 14 - 16 mg/dL, accompanied by acute oliguric kidney injury and altered mental status. In infants, common symptoms include poor feeding, failure to thrive, and hypotonia. Cognitive impairments, seizures, and optic nerve damage may occur as a result of persistent hypercalcemia. Psychological manifestations such as depression, confusion, dementia, decreased consciousness, and psychosis are also associated with hypercalcemia (1, 2, 13).
4.4. Overview of Hypercalcemia Causes in Pediatric Patients
As previously discussed, the majority of hypercalcemia cases (80 - 90%) fall into two etiological categories: Hypercalcemia associated with hyperparathyroidism and hypercalcemia associated with malignancy. Patients with hyperparathyroidism-related hypercalcemia are typically asymptomatic and most often discovered through elevated serum calcium levels during routine screenings. In contrast, patients with malignancy-associated hypercalcemia usually present as clinically ill and often arrive at the emergency department with acute symptomatic hypercalcemia (2, 14).
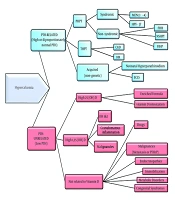
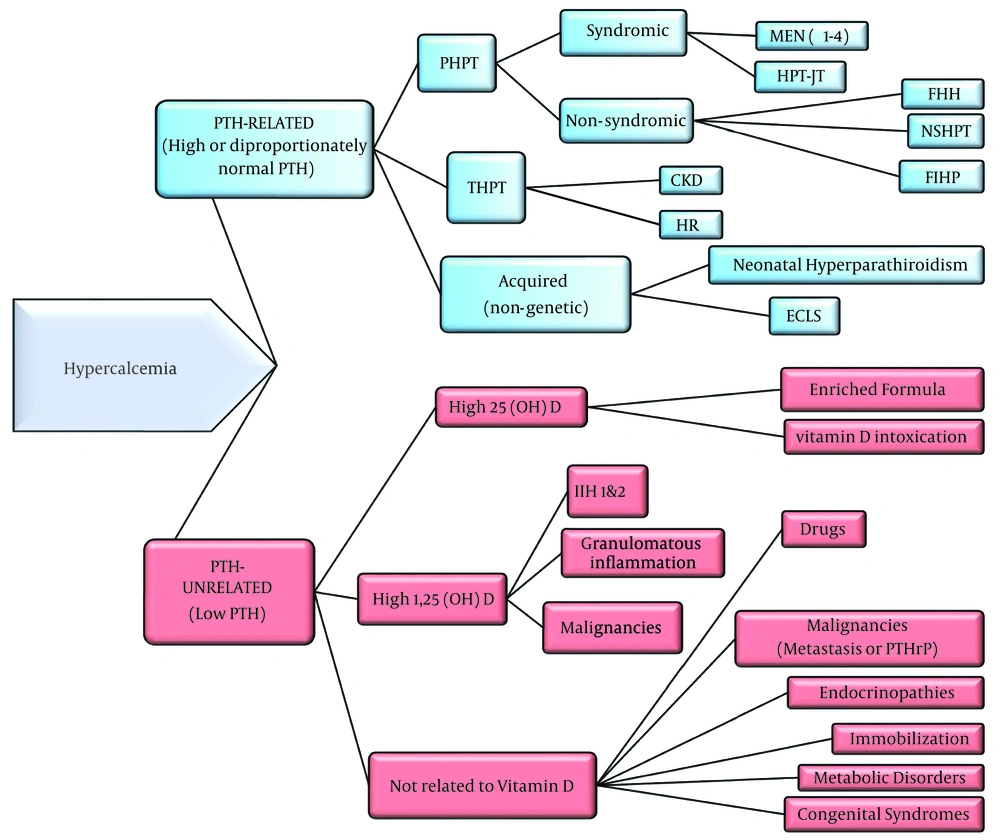
To categorize the causes of hypercalcemia, they are divided into two groups: PTH-related and PTH-unrelated. PTH-related hypercalcemia is identified by an increased or disproportionately normal level of PTH concurrent with hypercalcemia. In contrast, PTH-unrelated hypercalcemia, which is more common in pediatrics, is characterized by low PTH levels and can be attributed to numerous inherent or acquired causes (Figure 1).
Algorithm of Hypercalcemia Etiologies. Primary hyperparathyroidism, Primary Hyperparathyroidism; THPT, Tertiary Hyperparathyroidism; MEN, Multiple Endocrine Neoplasia; HPT-JT, Hyperparathyroidism-Jaw Tumor; FHH, Familial Hypocalciuric Hypercalcemia; NSHPT, Neonatal Severe Hyperparathyroidism; FIHP, Familial Isolated Hyperparathyroidism; CKD, Chronic Kidney Disease; HR, Hypophosphatemic Rickets; ECLS, Extracorporeal Life Support; IIH, Idiopathic Infantile Hypercalcemia; PTHrP, PTH-related Peptide.
4.4.1. PTH-Related Hypercalcemia
As previously mentioned, in this category of hypercalcemia, the serum PTH levels are high or disproportionately normal relative to the elevated serum calcium concentrations. Causes include PHPT encompassing both syndromic and non-syndromic causes, tertiary hyperparathyroidism (THPT) resulting from either CKD or Hypophosphatemic Rickets (HR), and acquired (non-genetic) neonatal hypercalcemia due to maternal hypocalcemia and extracorporeal life support (ECLS), with the latter two being the main causes not related to parathyroid tumors (1).
4.4.1.1. Primary Hyperparathyroidism
Primary hyperparathyroidism may manifest as either a hereditary disorder or a sporadic disease resulting from a de novo mutation. Both scenarios can increase the likelihood of inheriting PHPT in offspring (15).
Genetic-related causes of PHPT can be divided into syndromic and non-syndromic etiologies, as discussed below.
Syndromic causes of Primary Hyperparathyroidism (PHPT):
Multiple endocrine neoplasia: Multiple Endocrine Neoplasia (MEN) is a disorder characterized by an autosomal dominant inheritance pattern, presenting with two or more concurrent endocrine tumors, with four types recognized previously. Each type of MEN can present with a distinct group of tumors, and parathyroid tumors can be observed in all four types (16).
Parathyroid tumors are found in up to 95% of patients with MEN1, with hypercalcemia being the initial manifestation in 90% of these patients. It is important to note that MEN1 affects all four parathyroid glands and typically presents as a multigland disease. This implies that in patients who undergo subtotal parathyroidectomy, hypercalcemia may recur in the future due to the involvement of the remaining glands (17).
In MEN2, parathyroid tumors are less common compared to MEN1 (~20% of patients), with the two most significant tumors in this syndrome being medullary thyroid carcinoma (~99% of patients) and pheochromocytoma (~50%) (18).
Parathyroid tumors are extremely rare in MEN3, and although only a few cases of MEN4 have been described, all had parathyroid tumors (16).
Hyperparathyroidism-jaw tumor syndrome: Hyperparathyroidism-Jaw Tumor (HPT-JT) results from a germline mutation of the HRPT2 gene, which encodes a protein called parafibromin or Cell division cycle 73 (CDC73) with antiproliferative characteristics. This syndrome includes parathyroid adenomas, jaw tumors, and neoplasms of other organs, such as the kidneys and uterus. This mutation follows an autosomal dominant inheritance pattern, making a positive family history a crucial diagnostic clue. The hypercalcemia in this syndrome is more severe than in MEN1 or other non-syndromic parathyroid adenomas, necessitating early detection and treatment. The most common presentation is a single parathyroid adenoma, with parathyroidectomy often being curative. Patients with HPT-JT may also have associated uterine and renal tumors. The second most common manifestation of HPT-JT are uterine tumors, both benign and malignant, found in up to 50% of women with the CDC73 mutation. Due to the early onset of parathyroid tumors in this syndrome, biochemical screening for PHPT, starting as early as 5 - 10 years of age, is recommended for individuals with a positive family history (19).
Non-syndromic causes of Primary Hyperparathyroidism (PHPT):
Familial hypocalciuric hypercalcemia: Familial hypocalciuric hypercalcemia results from three gene mutations: Inactivating mutations of the CaSR (CASR) in FHH1, which is the most common form, mutations of the G-protein subunit α11 (GNA11) in FHH2, and mutations of the Adaptor protein-2 sigma subunit (AP2S1) resulting in FHH3. Approximately 65% of cases in the literature are identified as FHH1 with CASR mutations. Diagnosis for cases of FHH2 and FHH3 is usually confirmed when gene sequencing reveals GNA11 and AP2S1 gene mutations, respectively. Unlike other hypercalcemic conditions, FHH is generally benign and often diagnosed in asymptomatic patients during routine screenings. It is crucial to differentiate between PHPT, which requires parathyroidectomy, and FHH, which does not require treatment, to avoid unnecessary surgical intervention. Measuring the urine calcium/creatinine ratio is instrumental in distinguishing PHPT from FHH. Hypocalciuria is a significant indicator of FHH; thus, a 24-hour urine calcium and creatinine measurement should be included in the initial workup for hypercalcemia. If low or normal lower limit urine calcium is detected in a patient with hypercalcemia, FHH should be suspected (20).
Neonatal severe primary hyperparathyroidism: Neonatal severe primary hyperparathyroidism (NSHPT) is a rare and severe disorder characterized by pronounced hypercalcemia, respiratory distress, hypotonia, and bone demineralization shortly after birth. NSHPT is typically lethal within three months if untreated. It occurs when a loss of function mutation in the CaSR gene is present. NSHPT may also occur in offspring of FHH patients who receive two abnormal copies of the CASR gene, from both parents with FHH, or one mutated copy along with a de novo germline mutation. The treatment of choice, which is lifesaving, involves surgical removal of the affected parathyroid glands as soon as possible (21).
Familial isolated primary hyperparathyroidism: Familial Isolated Primary Hyperparathyroidism (FIHP) is considered a hereditary form of PHPT that occurs without other endocrine tumors. It is a diagnosis of exclusion; therefore, screening for other hereditary PHPT syndromes and testing for associated genes like MEN1 (MEN1 gene), MEN2 (RET gene), MEN4 (CDKN1B gene), HPT-JT (CDC73 gene), and FHH (CASR gene) should be performed. In many patients with FIHP, the genetic cause remains unidentified. Additionally, there is a hypothesis that FIHP may represent an incomplete expression of a syndromic PHPT, as some patients initially diagnosed with FIHP later develop features characteristic of MEN1 (1, 22).
4.4.1.2. Acquired (Non-genetic) Causes of Hypercalcemia in Neonates
Neonatal hyperparathyroidism: Maternal hypocalcemia, due to causes such as vitamin D deficiency, hypoparathyroidism, or pseudohypoparathyroidism, can provoke a compensatory increase in neonatal parathyroid hormone (PTH) secretion, known as neonatal hyperparathyroidism. This condition is typically temporary and usually resolves within a few weeks (23).
Extracorporeal life support: Hypercalcemia is a potential complication in neonates undergoing extracorporeal life support (ECLS). The exact cause of hypercalcemia associated with ECLS is not well understood; however, it may be related to changes in circulating PTH. Hypercalcemia has been commonly observed in neonates requiring extended durations of ECLS. The clinical significance and underlying etiology of this phenomenon remain unclear and warrant further investigation (24).
4.4.1.3. Tertiary Hyperparathyroidism
Tertiary hyperparathyroidism is a condition characterized by the autonomous hypersecretion of PTH, often due to hyperplasia of a group of parathyroid cells that possess a higher set point of the CaSR as a result of a preexisting comorbidity. This results in continued PTH secretion despite elevated serum calcium levels. THPT is most commonly seen in patients with longstanding CKD and often persists after renal transplantation when renal function normalizes but PTH levels remain elevated (25).
Tertiary hyperparathyroidism is also a rare complication in patients with hypophosphatemic rickets (HR), typically arising from long-term treatment with active forms of vitamin D and oral phosphate supplements. If left untreated, THPT may worsen renal function and exacerbate bone complications (26).
4.4.2. PTH-Unrelated Hypercalcemia
PTH-unrelated hypercalcemia, more prevalent in pediatric patients, can arise from various inherent or acquired sources and can be categorized as follows:
(1) Hypercalcemia with high serum 25-hydroxyvitamin D3 (25(OH)D3)
(2) Hypercalcemia with high serum 1,25-dihydroxyvitamin D3 (1,25(OH)2D3)
(3) Hypercalcemia not associated with vitamin D
4.4.2.1. Hypercalcemia with High Serum 25(OH)D3
Vitamin D intoxication, due to incorrect prescription dosages (e.g., enriched formulas) or accidental overdosing, is a well-recognized cause of hypercalcemia. The precise mechanism by which elevated levels of 25(OH)D3 induce hypercalcemia is still debated. It is hypothesized that high concentrations of 25(OH)D3 in the bloodstream may displace 1,25(OH)2D3 from its binding protein, thereby increasing the level of free 1,25(OH)2D3. This unbound 1,25(OH)2D3 can then stimulate gene transcription, leading to hypercalcemia (27).
4.4.2.2. Hypercalcemia with High Serum 1,25(OH) D3
Increased levels of 1,25(OH)2 D3 may be due to various factors:
- Decreased renal degradation of 1,25(OH)2 D3 to inactive 24,25(OH)2 D3, as observed in idiopathic infantile hypercalcemia (IIH Type 1).
- Overproduction in renal tissue due to phosphate depletion, noted in IIH Type 2.
- Overactivity of the 1α-hydroxylase enzyme in extrarenal tissues, commonly seen in conditions involving granulomatous inflammation and malignancies.
Idiopathic infantile hypercalcemia (IIH type 1 & 2): Phosphorus is essential for metabolism, notably in ATP synthesis, phosphorylation of key enzymes, and its buffering role in acid-base balance. Additionally, phosphorus is a component of hydroxyapatite (HA), a crucial mineral in bone structure (28).
The FGF23 is pivotal in vitamin D metabolism. FGF23 inhibits renal 1α-hydroxylase (CYP27B1)—the enzyme responsible for increasing active vitamin D production—and stimulates 1,25-dihydroxyvitamin D-24-hydroxylase (CYP24A1), which promotes the deactivation of active vitamin D. Phosphate is reabsorbed in the proximal tubules of the kidney by sodium-phosphate cotransporters, primarily types 2A (NaPi-IIa) and 2C (NaPi-IIc). The function of NaPi-IIa is notably influenced by FGF23 and parathyroid hormone (PTH) (29).
Idiopathic Infantile Hypercalcemia typically presents within the first year of life with symptoms such as hypercalcemia, impaired growth, dehydration, vomiting, and stupor, and can be fatal. IIH has an autosomal recessive inheritance pattern. There are two recognized forms of IIH—IIH1 and IIH2—attributable to mutations in the genes CYP24A1 (encoding 1,25-dihydroxyvitamin D-24-hydroxylase) and SLC34A1 (encoding the sodium-phosphate cotransporter 2A, NaPi-IIa), respectively (30).
A mutation in the SLC34A1 gene leading to defective NaPi-IIa activity results in phosphorus depletion and subsequent decreased circulating FGF23 levels. As FGF23 levels decrease, the inhibition of the 1α-hydroxylase enzyme imposed by FGF23 is lifted, resulting in inappropriate and excessive production of active vitamin D. This excessive vitamin D activity leads to hypercalcemia, increased urinary calcium excretion, and calcium deposition in the kidneys, typical of idiopathic infantile hypercalcemia Type 2 (IIH Type 2) (31).
Overactivity of 1α-hydroxylase enzyme in extrarenal tissues: Certain malignancies, such as non-Hodgkin and Hodgkin lymphomas, and ovarian dysgerminomas, can cause hypercalcemia by increasing the production of 1,25(OH)2D3 due to their ability to express high levels of 1α-hydroxylase (32).
Macrophages can also act as an extrarenal site with a considerable amount of 1α-hydroxylase activity (33). In conditions with granulomatous inflammation such as sarcoidosis, tuberculosis, fungal infections like coccidioidomycosis and histoplasmosis, cat scratch disease, Crohn’s disease, cytomegalovirus (CMV) infection, and subcutaneous fat necrosis of the newborn (SFN), macrophage activity can lead to increased extrarenal production of 1,25(OH)2D3, resulting in subsequent hypercalcemia (34).
Subcutaneous fat necrosis of the newborn (SFN) is a rare condition resulting from granulomatous inflammation in the subcutaneous fat tissue. Lesions of SFN usually appear as single or multiple erythematous plaques or nodules that can evolve into calcifications. These lesions are most commonly found on the face, back, shoulders, and buttocks. SFN occurs following a history of an insult such as labor trauma, asphyxia, hypothermia, or therapeutic cooling (i.e., in hypoxic-ischemic encephalopathy treatment) and may appear from birth until the first six weeks afterward. This condition is associated with life-threatening hypercalcemia, and the extent of skin involvement correlates with the severity and duration of the hypercalcemia. The mechanism explaining hypercalcemia in SFN is that an insult to the adipocytes, such as exposure to cold, leads to necrosis and subsequent granulomatous inflammation. The presence of macrophages in the granulomatous infiltrate, with an abundant level of 1α-hydroxylase activity, results in increased production of 1,25(OH)2D3 and hypercalcemia, similar to other granulomatous disorders (35).
4.4.2.3. Hypercalcemia Not Associated with Vitamin D
PTH-unrelated hypercalcemia can occur without changes in circulating vitamin D concentrations. The etiologies for this form of hypercalcemia are diverse and include:
Drugs: Thiazides act as hypocalciuric agents and are therefore potential candidates to trigger hypercalcemia or to uncover a previously compensated hypercalcemia caused by other etiologies (36).
Vitamin A (retinol) and its derivatives (e.g., isotretinoin) are used to treat various disorders, such as acne vulgaris and hematologic disorders, and can cause hypercalcemia by increasing osteoclast activity and bone resorption (37).
Prolonged use of lithium is a well-established risk factor for hypercalcemia. Studies suggest that lithium raises the threshold level of calcium required to suppress PTH by antagonizing the CaSR, thereby leading to an elevated PTH level and subsequent hypercalcemia (38, 39).
Malignancy: Hypercalcemia associated with malignancy may occur through three different mechanisms: Osteolytic metastases or infiltration by leukemias; bone resorption induced by PTH-related peptide (PTHrP); and an increased production of 1,25(OH)2 D3, as previously discussed. In normal bone physiology, PTHrP acts as a local hormone regulating skeletal development. However, certain solid tumors such as renal cell carcinoma, squamous cell carcinoma, ovarian and breast carcinomas, neuroblastoma, dysgerminoma, rhabdomyosarcoma, and pheochromocytoma may secrete PTHrP, leading to hypercalcemia (40, 41).
Endocrinopathies: Hypercalcemia can occur in various endocrine disorders, including pheochromocytoma, primary adrenal insufficiency (Addison's disease), thyrotoxicosis, and congenital hypothyroidism.
Pheochromocytoma, a rare tumor that secretes catecholamines, is the most common neuroendocrine tumor in children, with 70% being unilateral and confined to the adrenals. It is more commonly associated with familial syndromes than sporadic cases. The most notable mutations that cause this tumor include RET (MEN2) and VHL (Von Hippel-Lindau syndrome), which cause bilateral pheochromocytomas in families with these syndromes (42). Pheochromocytoma may also lead to hypercalcemia by secreting PTHrP (39).
Addison's disease can cause hypercalcemia, potentially due to decreased renal calcium excretion (43).
Thyroid hormones increase bone resorption by enhancing osteoclast activity; thus, thyrotoxicosis can disrupt the balance between bone deposition and resorption in children, possibly leading to hypercalcemia (44).
Hypercalcemia is also seen in neonates with severe congenital hypothyroidism, although it is usually not clinically significant and occurs with an unclear mechanism. Interestingly, hypercalcemia was only observed in neonates with this condition who were receiving levothyroxine and vitamin D. However, hypercalcemia did not seem to be related to vitamin D levels, which were not elevated compared to unaffected neonates. Increased sensitivity of target tissues to normal levels of 1,25(OH)2D3 might explain the hypercalcemia in these cases (45).
Immobilization: Immobilization hypercalcemia typically occurs within the first four to six weeks after an injury that results in the patient becoming bedridden. This condition is less common in pediatrics and is usually found in adolescents. In this scenario, elevated ionized and total calcium levels are associated with low levels of PTH, 25(OH) D3, and 1,25(OH)2 D3. The hypothesized mechanism suggests that immobilization increases osteoclast activity, though the exact mechanism remains unclear. Additionally, immobilization decreases the glomerular filtration rate, which could contribute to subsequent hypercalcemia development (46).
Inborn errors of metabolism: Several metabolic disorders can cause hypercalcemia, including:
Hypophosphatasia (HPP) is an Inborn Errors of Metabolism (IEM) characterized by deficient bone mineralization due to a defective or absent alkaline phosphatase enzyme, caused by mutations in the tissue-nonspecific alkaline phosphatase (TNALP) gene. Patients with HPP often experience hypercalcemia, alongside characteristic skeletal abnormalities. It is suggested that hypercalcemia results from impaired bone mineralization by osteoblasts while osteoclasts continue normal bone resorption (47).
Congenital lactase deficiency (CLD) is a rare autosomal recessive disease. Its hallmark symptoms, including loose diarrhea beginning shortly after initiating breastfeeding, make it a recognizable condition if suspected. Affected neonates are typically alert, with good appetites and no vomiting or weight loss. Hypercalcemia is so pronounced in these patients that it can be a diagnostic clue for CLD in infants presenting with persistent diarrhea and failure to thrive. The exact mechanism of hypercalcemia in CLD is unknown, but it is thought that chronic metabolic acidosis, common in CLD, significantly affects calcium levels by using bone minerals to buffer acid. Another explanation suggests that dietary lactose directly increases calcium absorption in the ileum, independent of vitamin D activity (48, 49).
Disaccharide intolerance disorders such as sucrose-isomaltase deficiency may cause hypercalcemia with similar pathophysiology to CLD (50).
Blue diaper syndrome, an extremely rare disorder caused by errors in tryptophan metabolism, has also been associated with hypercalcemia through unknown mechanisms (51).
Congenital syndromes: Hypercalcemia in pediatric patients is often attributed to genetic etiologies and may be part of a congenital syndrome. These disorders are usually identified early in life, although in some cases, the syndromic appearance and dysmorphism may be subtle or overlooked, making diagnosis challenging based solely on the child's age (1).
Williams Syndrome results from the deletion of up to 28 genes on chromosome 7q. It is characterized by distinctive facial features, learning difficulties, developmental delays, a sociable personality, cardiovascular anomalies such as aortic or pulmonic valve stenosis, nephrocalcinosis, genitourinary anomalies, and endocrinopathies, including hypercalcemia, which occurs in up to 43% of patients. The exact mechanism of hypercalcemia is unclear, but it may involve abnormal 1,25(OH)2D3 metabolism and reduced production of calcitonin (52, 53).
Jansen's metaphyseal chondrodysplasia (JMC) is a rare disorder characterized by abnormal bone deposition and severe hypercalcemia with normal or low levels of PTH and PTHrP. Both PTH and PTHrP activate a G protein-coupled receptor called the PTH type 1 receptor (PTH1R). In JMC, activating mutations of this receptor result in hypercalcemia accompanied by low levels of PTH and vitamin D (54).
Hypercalcemia has also been observed in individuals with Down syndrome, alongside nephrocalcinosis. This is an uncommon occurrence with only six previous case reports documented. Typically presenting in early life, this condition has been termed the ABCD syndrome (AB-normal calcium, calcinosis, creatinine in Down syndrome). The mechanism behind hypercalcemia in this syndrome is unclear, but the condition responds well to dietary calcium restriction, suggesting that it may stem from increased intestinal calcium absorption (55).
Cystinosis, which can manifest at any age but is most commonly seen in its infantile or early-onset form, has also been associated with hypercalcemia. Although rare, hypercalcemia in cystinosis may result from bone resorption or increased 1α-hydroxylase activity due to phosphorus depletion (56, 57).
4.5. Overview of Hypercalcemia Management
Managing hypercalcemia involves two main strategies: Firstly, reducing serum calcium levels to minimize effects on cell function, particularly in cardiac cells, neurons, and muscle cells; and secondly, identifying and addressing the underlying cause of elevated serum calcium (1).
A low-calcium diet is recommended for all patients with hypercalcemia, particularly those with mild hypercalcemia who are candidates for outpatient management and less aggressive treatments. This diet restricts dairy products such as cheese, milk, yogurt, and ice cream. It also involves the withdrawal of calcium and vitamin D supplements and reducing sunlight exposure, which can elevate vitamin D levels. Additionally, ketogenic diets, which have been linked to the development of hypercalcemia, should be avoided in these patients (58).
It is also wise to first discontinue medications that may exacerbate hypercalcemia, such as calcium and vitamin D supplements, along with other drugs previously discussed. Before initiating treatment, these diagnostic tests can help identify the underlying etiology: Serum corrected calcium, phosphate, albumin, vitamin D, PTH, alkaline phosphatase, creatinine, magnesium, electrolytes, 24-hour urine calcium and creatinine, an ABG to assess acid-base status, and saving serum for later evaluation of 1,25 (OH)2 D3 and PTHrP (1).
IV hydration is the initial treatment step for a symptomatic patient with hypercalcemia. Fluid therapy compensates for sodium and water deficits and stimulates diuresis, facilitating calcium removal. The choice of solution for this purpose is a subject of debate; however, considering the volume depletion and the role of sodium in calcium transport in proximal tubules, 0.9% normal saline is the best option because it contains more sodium than other isotonic solutions. Contrary to earlier studies that considered furosemide a standard treatment for hypercalcemia, more recent studies suggest that the effects of furosemide on calcium diuresis are limited, and there is also a risk of worsening the patient's dehydration and electrolyte imbalances; therefore, furosemide should be used cautiously to avoid deteriorating the patient's fluid and electrolyte status (59).
Subcutaneous calcitonin (4 - 8 IU/kg) is a useful anti-osteoclast drug for symptomatic hypercalcemia; however, due to tachyphylaxis, its effectiveness is short-term. In contrast, intravenous bisphosphonates (e.g., pamidronate 0.5 mg/kg) have a slower onset of action but provide more powerful and prolonged inhibition of bone resorption, making them the preferred choice for both initial and ongoing management of hypercalcemia. It is essential to assess the patient’s hydration status before starting treatment with IV bisphosphonates due to the potential nephrotoxicity of these agents (60, 61).
Glucocorticoids are effective for treating hypercalcemia associated with granulomatous disorders and malignancies, but they should be used cautiously due to their potential to increase the risk of osteoporosis and bone fragility (62).
Cinacalcet, a calcimimetic CaSR modulator, is used for the temporary management of hypercalcemia in NSHPT before surgery. However, the efficacy of cinacalcet likely depends on the residual functionality of the CaSR, and patients with homozygous CaSR mutations usually will not respond to this agent (63).
Hemodialysis with calcium-free dialysate is a treatment option reserved for patients who do not respond to IV hydration and the medications mentioned previously or for those in whom IV hydration, with or without furosemide diuresis, cannot be performed optimally due to pre-existing conditions such as chronic heart failure (CHF) or CKD (64).
Finally, parathyroidectomy is the curative procedure and the most appropriate treatment for children with PHPT or NSHPT (65).
5. Conclusions
Hypercalcemia is an important electrolyte disturbance that may be less common in pediatric patients than in adults, but it holds the same level of importance in terms of thorough workup and treatment as it can cause cell action potential disturbances resulting in catastrophic effects on all tissues, especially the cardiac, musculoskeletal, and nervous systems. Before initiating the workup for hypercalcemia, it is crucial to have a comprehensive understanding of calcium physiology and the factors affecting its serum concentrations, especially factors causing pseudohypercalcemia, which, if not accurately identified, may lead to mismanagement in various diagnostic and treatment aspects.
It is important to consider that genetic and syndromic causes of hypercalcemia are usually more prevalent in pediatrics due to the nature of these conditions presenting at birth or early in life; however, age itself should not be the sole factor in ruling out a condition in the workup of hypercalcemia. This article provides a concise overview of calcium metabolism and hypercalcemia in pediatric patients, highlighting the importance of recognizing the diverse etiologies and age-related differences in diagnosis and management.