1. Background
Immunoglobulin A (IgA)-mediated vasculitis, previously recognized as Henoch-Schönlein Purpura (HSP), is the most common vasculitis in children. It is a leukocytoclastic vasculitis caused by the accumulation of IgA in the small vessels of the skin, joints, gastrointestinal (GI) system, and kidneys. Henoch-Schönlein Purpura presents with skin lesions such as palpable purpura, musculoskeletal manifestations such as arthritis and arthralgia, GI manifestations such as abdominal pain, diarrhea, vomiting, and ileus, renal manifestations such as hematuria, proteinuria, hypertension, and rarely nephrotic and nephritis syndrome, and neurological manifestations such as headache, seizure, cerebral hemorrhage, and behavioral changes (1, 2). Renal involvement is reported variably in 20 to 80% of HSP patients, and GI involvement is found in 50 to 63% (2-4).
Calprotectin, or myeloid-related protein (MRP) 8/14, is a calcium-binding protein mainly found in neutrophils and macrophages. Calprotectin levels correlate with neutrophil accumulation and increase in settings of inflammation (5). There is a confirmed relationship between fecal calprotectin and inflammatory GI diseases such as Crohn's disease and ulcerative colitis (6-8). Fecal calprotectin is considered a better indicator of the possibility of inflammatory bowel disease (IBD) than serum inflammatory markers (9). Calprotectin levels have also been associated with disease severity in renal disorders such as acute kidney injury (AKI) and nephrotic disease (10, 11). New implications have also been found for calprotectin levels in rheumatologic diseases (12, 13). Elevated levels of calprotectin were found in patients with rheumatoid arthritis, consistent with disease activity (14).
A few studies have investigated the role of calprotectin in HSP. Serum calprotectin levels have been associated with clinical and pathological symptoms of HSP nephritis (15). Furthermore, some studies have proposed that fecal calprotectin levels can indicate early GI involvement in HSP (16, 17).
2. Objectives
Since the association between calprotectin and HSP complications is not well discussed in the literature, we decided to conduct a study to investigate the role of calprotectin in predicting HSP complications. We aim to determine if calprotectin can be used as a determinant factor in the follow-up program of HSP patients.
3. Methods
Patients diagnosed with HSP by the EULAR/PRINTO/PRES criteria who were admitted to the rheumatology wards of Children’s Medical Center and Bahrami Children’s Hospital in Tehran, Iran, were eligible for inclusion in our study. A patient is classified as having HSP according to these criteria when there is purpura or petechiae with lower limb predominance, without evidence of coagulopathy or thrombocytopenia, plus one of the following four criteria: (1) abdominal pain; (2) arthritis or arthralgia; (3) renal involvement; and (4) evidence of IgA deposition in histopathology (18).
The following patients were excluded: (1) patients admitted with a suspicion of HSP but later diagnosed with another disease; (2) patients with an inflammatory or autoimmune underlying disease of the GI tract, such as familial Mediterranean fever (FMF) or inflammatory bowel disease (IBD); (3) patients with a previous chronic renal disease; and (4) patients with chronic GI tract diseases such as food allergy.
To determine the sample size, we needed to know the prevalence of positive calprotectin in HSP, which was not available due to the few studies on this topic. Therefore, we used the studies of Teng et al. (17), Kanik et al. (16), and Kawasaki et al. (15). The mean sample size in these studies was 50. Anticipating potential difficulties in obtaining fecal samples from admitted patients and the possibility of losing some enrolled patients, we enrolled twice this number, totaling 100.
The patients’ fecal calprotectin levels were measured on the first day of admission using the Biohit Calprotectin ELISA kit, Biohit Healthcare. All samples were tested in the same laboratory. A high level of calprotectin was defined as higher than 100 µg/g in children under 4 years old and higher than 50 µg/g in children older than 4 years old. The patients were then followed for GI and renal complications that occurred during admission and in monthly follow-ups for up to three months. Renal complications were defined as hematuria or proteinuria found in urine analysis (U/A), and GI complications were defined as any level of GI bleeding, including positive occult blood (OB) test, abdominal pain starting at least one month prior to the appearance of skin lesions, elevated liver enzymes, or evidence of intussusception on sonography. We also analyzed the relationship between fecal calprotectin and leukocyte count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) levels. The data were analyzed using SPSS 24.
We obtained consent from the patients’ parents for participation in our study. The study adhered to the tenets of the Declaration of Helsinki and the ethical committee of Tehran University of Medical Sciences (TUMS); ethical code available at: IR.TUMS.CHMC.REC.1398.052.
4. Results
Among the patients who fulfilled the HSP criteria, one patient was excluded as he was later diagnosed with systemic lupus erythematosus. Additionally, 52 patients did not provide a fecal sample while admitted to the hospital, leaving 47 patients enrolled in the analysis. Seventy-five percent of the patients were boys. The age of the patients ranged from 2 to 18 years, with a mean of 6.5 ± 2.9 years.
Abdominal pain was reported in 80% of the patients, and OB was found in 6.4%. Intussusception was not reported in any of the patients. Hematuria was present in 21% of the patients at the time of disease presentation, and proteinuria was found in 17%. The quantity of hematuria and proteinuria was classified on a scale from 1 to 4.
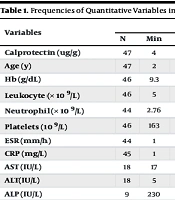
Abdominal sonography was performed when a patient had abdominal pain. A small amount of interloop liquid was reported in three patients, mesenteric lymphadenitis (up to 7 mm) was reported in three patients, increased thickness of the intestinal wall was reported in two patients, and severe edema in the scrotum was reported in one patient. Liver function tests were checked in 17 patients, and they were elevated in only one patient. The frequencies of quantitative variables are summarized in Table 1.
| Variables | Total | Normal Calprotectin Group | High Calprotectin Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Min | Max | Median | Mean | N | Min | Max | Median | Mean | N | Min | Max | Median | Mean | |
| Calprotectin (ug/g) | 47 | 4 | 1375 | 45 | - | 25 | - | - | - | - | 22 | - | - | - | - |
| Age (y) | 47 | 2 | 18 | - | 6.5 | 25 | 3 | 18 | - | 6.9 | 22 | 2 | 12 | - | 6.11 |
| Hb (g/dL) | 46 | 9.3 | 15.7 | - | 12.5 | 25 | 9.3 | 14.4 | - | 12.2 | 21 | 9.9 | 15.7 | - | 12.85 |
| Leukocyte (× 109/L) | 46 | 5 | 22.6 | - | 12.3 | 25 | 5 | 18.97 | - | 11.59 | 21 | 5.69 | 2.96 | - | 13.196 |
| Neutrophil (× 109/L) | 44 | 2.76 | 18.2 | - | 8.4 | 25 | 3.27 | 16.58 | - | 7.48 | 19 | 2.76 | 18.24 | - | 9.635 |
| Platelets (109/L) | 46 | 163 | 1023 | - | 374 | 25 | 202 | 512 | - | 351 | 21 | 163 | 1022 | - | 425 |
| ESR (mm/h) | 44 | 1 | 87 | 22.5 | - | 25 | 4 | 87 | 14 | - | 19 | 1 | 52 | 15 | - |
| CRP (mg/L) | 45 | 1 | 120 | 18.8 | - | 25 | 1 | 56 | 5 | - | 20 | 1 | 120 | 9 | - |
| AST (IU/L) | 18 | 17 | 96 | 28.7 | - | 8 | 21 | 96 | 26 | - | 10 | 17 | 49 | 22 | - |
| ALT(IU/L) | 18 | 5 | 74 | 12 | - | 8 | 9 | 62 | 14 | - | 10 | 5 | 74 | 11 | - |
None of the patients reported abdominal pain or other GI symptoms at the 3-month follow-up. Sixty-six percent of the patients (31 patients) underwent urine analysis at the three-month follow-up, and only three patients had abnormal urine results. The results of the urine analysis at the three-month follow-up are summarized in Table 2.
| Patient | Hematuria at Onset | Hematuria at Follow-up | Proteinuria at Onset | Proteinuria at Follow-up |
|---|---|---|---|---|
| A | 0 | 0 | 1+ | 1+ |
| B | 3+ | 3+ | 0 | 0 |
| C | 4+ | 2+ | 4+ | 1+ |
Calprotectin levels ranged from 4 to 1375 µg/g, with a median of 45 µg/g. Overall, 53% of the patients were categorized as having low calprotectin levels, and 47% as having high levels.
The Kolmogorov-Smirnov test showed that the distribution of calprotectin levels was abnormal; therefore, we used non-parametric tests to find the associations between calprotectin levels and other variables. The Mann-Whitney test found an association between calprotectin levels and blood in the stool (P = 0.03) but did not find any association between calprotectin levels and gender, hematuria, proteinuria, abdominal pain, or abnormal abdominal sonography (Table 3).
The Pearson correlation test found a positive correlation between calprotectin levels and leukocyte count (P = 0.003), neutrophil count (P = 0.002), and CRP (P = 0.03) (Table 4).
| Mann-Whitney Test | Calprotectin Level | |||
|---|---|---|---|---|
| Mann-Whitney U | Wilcoxon W | Z | P-value | |
| Blood in stool | 19.00 | 1009 | -2.04 | 0.03 |
| Hematuria | 120.5 | 165.5 | -1.36 | 0.17 |
| Proteinuria | 128.00 | 173.00 | -1.16 | 0.25 |
| Abdominal pain | 151.5 | 196.5 | -0.52 | 0.60 |
| Abnormal sonography | 123.50 | 943.50 | -0.49 | 0.62 |
a P < 0.05 considered statistically significant.
b P < 0.005 considered statistically significant.
5. Discussion
The current study investigates the fecal calprotectin levels of 47 HSP patients and their association with renal and GI complications of HSP. The fecal calprotectin test is a simple, non-invasive test, and confirming its value in predicting morbidities in HSP can lead to prompt interventions. However, this test is expensive, and its cost-utility needs to be thoroughly examined.
In the retrospective study by Paek et al. in 2020, blood calprotectin levels of 69 HSP patients were tested. Gastrointestinal symptoms were defined as abdominal pain, vomiting, bloody stool, and GI complications on imaging. The 40 patients who had GI symptoms had significantly higher calprotectin levels (19). The cohort study by Teng et al. in 2015 reported that the fecal calprotectin levels of 40 HSP patients with GI symptoms were significantly higher than those of 40 other HSP patients without GI symptoms. The GI symptoms included abdominal pain (100%), diarrhea (10%), constipation (27%), and bloody stool (12.5%) (17). We reported bloody stool in 6.4% of the patients, close to the rates reported by Teng (12.5%) and Kanik (10.6%) (16), and found it to be associated with calprotectin levels in the patients. However, abdominal pain and abnormal abdominal sonography findings were not associated with calprotectin levels. The studies by Teng and Paek combined all the GI symptoms and then analyzed their association with calprotectin levels. In contrast, we analyzed the associations of each sign/symptom individually and found an association only with bloody stool. Studying larger populations with higher numbers of abnormal imaging might lead to finding significant associations.
Wang et al. studied 61 HSP patients with GI involvement in 2020 in China and found two cases with intussusception (20). Moreover, Dörterler et al. conducted a retrospective study on 183 cases of intussusception and reported HSP vasculitis as a possible cause for intussusception (21). We did not find any cases of intussusception in our 47 HSP patients; however, the previous findings suggest that we should be vigilant for this serious condition in HSP patients with abdominal pain.
Rosti et al. investigated 71 HSP patients for hepatic complications. Nine percent of the patients had mildly elevated liver enzyme tests, and all levels returned to normal after two to four weeks (22). We reported only one patient with mildly elevated AST and ALT. It can be suggested that hepatic involvement in HSP is rare, and routine testing may not be necessary.
Kanik et al. reported that the level of calprotectin was higher in patients with renal complications of HSP, such as hematuria, proteinuria, and elevated creatinine (16). Kawasaki et al. studied HSP patients with renal injury according to kidney biopsy in two groups: Low and high calprotectin. Proteinuria was more frequent in the high calprotectin group, but there was no significant difference in hematuria and creatinine levels. The frequency of international study of kidney disease in children (ISKDC) classification grades of 3, 4, or 5 was higher in the high calprotectin group (15). Ohara et al. divided 37 patients into two groups: Minimal change disease and glomerulonephritis (GN), and compared their levels of MRP8/14, which were significantly higher in the GN group (10). Conversely, we did not find any association between calprotectin level and hematuria or proteinuria. This might be due to the small sample size and therefore the low number of patients with hematuria (10 patients) and proteinuria (8 patients). Furthermore, there was no indication for a renal biopsy in our patients to analyze the association of pathology with calprotectin levels.
Teng et al. reported that leukocyte count and CRP are associated with calprotectin levels (17). Likewise, we found a significant association between calprotectin level and leukocyte count, neutrophil count, and CRP. The ROC curve in the study by Teng et al. showed that fecal calprotectin is more sensitive than leukocyte count in the early diagnosis of HSP with GI complications. Nevertheless, the high price of the calprotectin test increases the value of the complete blood count (CBC) test.
An important limitation of our study was the need for stool samples from patients upon admission. Many of our HSP patients did not provide a specimen during their admission, which was usually short, less than three days. This is why the number of included cases was less than what we had expected based on the number of HSP patients admitted to our center.
5.1. Conclusions
According to our study, positive blood in the stool is associated with fecal calprotectin levels in HSP patients. However, hematuria and proteinuria were not associated with calprotectin levels. Larger study populations might be able to find such an association; however, considering the high cost of the calprotectin test, monthly follow-up with urine analysis seems to be a more logical approach. Leukocyte count, neutrophil count, and CRP were found to be correlated with calprotectin levels, highlighting the nature of fecal calprotectin as an acute inflammatory marker and its elevation during the acute phase of HSP disease.