1. Background
Hypothyroidism can be present at birth (congenital) or diagnosed later in life (acquired). Congenital hypothyroidism affects approximately 1 in 2,000 neonates and can have adverse effects on neurodevelopment (1). Thyroxine (T4) is released from the thyroid gland and converted into triiodothyronine (T3), the biologically active form. Triiodothyronine plays crucial roles in brain neurodevelopmental processes, such as glial myelination, neuronal migration, cortical layer formation, synaptogenesis, and neurogenesis. Maternal hypothyroidism, depending on the severity and duration of hormone insufficiency, may cause congenital hypothyroidism. Congenital hypothyroidism affects the cerebral cortex and a range of brain functions (motor, cognition, attention, and personal-social skills), leading to long-lasting neurodevelopmental complications (2, 3). This relationship between thyroid hormone and a child's neurodevelopment highlights the importance of neonatal screening for hypothyroidism. Health care systems in most countries implement neonatal screening strategies to assess thyroid-stimulating hormone (TSH) levels during the neonatal period, with subsequent T4 measurement in cases with elevated TSH values (4).
Studies have shown delayed neurodevelopmental status in children with congenital hypothyroidism. Literature indicates that cognitive and communication skills in patients with congenital hypothyroidism are significantly lower than those of their healthy counterparts (5, 6). One investigation also found that language, fine motor, and gross motor domains were adversely affected by congenital hypothyroidism (7). The severity of hypothyroidism, age at the initiation of treatment, and drug dosage are the main factors influencing neurodevelopmental status (6). It has been demonstrated that starting treatment within the first two months of life can improve neurodevelopmental outcomes (7). However, this finding was not corroborated by another study, which reported mild to moderate neurodevelopmental delays in congenital hypothyroidism cases despite early treatment (8).
On the other hand, moderate elevations (5 - 15 mIU/L) in neonatal TSH and transient hypothyroxinemia have not been shown to affect intelligence quotient (IQ), motor function, or language skills (9, 10). Several studies have assessed neurodevelopmental outcomes in congenital hypothyroidism cases or compared their neurodevelopmental skills with those of healthy controls (11-13). To our knowledge, the neurodevelopmental status in children with acquired hypothyroidism is less well-known, and no studies have compared this variable between congenital and acquired hypothyroidism patients.
2. Objectives
However, in clinical practice, concerns regarding neurodevelopmental outcomes in hypothyroid infants with normal hypothyroidism screening test results persist. In light of this and the importance of early interventions, the present study was conducted.
3. Methods
A retrospective cohort study was conducted in the Pediatric Department of Imam Khomeini Hospital Complex, affiliated with Tehran University of Medical Sciences (Tehran, Iran), from March 2021 to March 2022. Children aged 3 - 5 years with a diagnosis of hypothyroidism, attending the outpatient endocrinology clinics of two tertiary referral centers (Vali-e-Asr and Ziaeean Hospitals), were included in the study. Based on their medical records, participants were divided into two groups: Congenital and acquired hypothyroidism. In the congenital group, the disease was diagnosed following the national neonatal screening program. The acquired hypothyroidism group consisted of children with normal neonatal screening results, whose disease manifested after the neonatal period. Both groups were receiving levothyroxine treatment.
Inclusion criteria included age between 3 and 5 years, a confirmed history of hypothyroidism, and ongoing medication for hypothyroidism. Exclusion criteria were age below 3 or above 5 years, a history of congenital or chromosomal complications, a history of neonatal complications such as preterm birth, asphyxia, sepsis, neonatal intensive care unit admission, childhood autoimmune or metabolic disorders, the use of medications other than levothyroxine that could affect thyroid function (such as corticosteroids), non-adherence to hypothyroidism treatment, and missing data regarding the time of disease diagnosis or treatment initiation.
The neurodevelopmental status of participants was determined using the Ages and Stages Questionnaire (ASQ). The ASQ-3 is a developmental screening tool consisting of 30 items, designed to assess five neurodevelopmental domains: Communication, gross motor, fine motor, problem-solving, and personal-social behavior (6 questions for each domain) in children aged one month to 5.5 years (14). Parents were asked to respond to each question using three options: "Yes" = 10; "Sometimes" = 5; and "Not yet" = 0. The scores for each domain, as well as the total score across all domains, were calculated. Each participant's total score was compared to the cutoff points for their respective age group to determine their neurodevelopmental status. Final scores above -1 SD and below -2 SD were classified as normal, while scores below -2 SD indicated abnormal neurodevelopmental status (14). Children with scores between -1 and -2 SD received a training package for two weeks, after which the questionnaire was completed again.
All data regarding children's gender, age, age at diagnosis, drug dosage at treatment onset, TSH and free thyroxine (FT4) levels at the time of diagnosis, as well as maternal history of hypothyroidism and drug consumption, were collected by referring to the patients' medical records. Additionally, the final ASQ scores for each domain were recorded. The frequency of abnormal neurodevelopmental status was then compared between the groups.
3.1. Ethical Consideration
The subject of this research and the methodology were approved by the Medical Ethics Committee of Tehran University of Medical Sciences (approval number: IR.TUMS.MEDICINE.REC.1399.107). The study protocol was conducted in accordance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects and adhered to the research regulations of the country.
3.2. Sample Size
According to a study by Perri et al. (15), children with congenital hypothyroidism had a lower mean cognitive score compared to their controls (92.18 ± 18.50 vs. 103.55 ± 12.44). Using the sample size formula to compare the mean values between independent populations, the sample size was calculated to be 28 in each group, considering an error margin of 0.05 and a power of 80%.
3.3. Statistical Analysis
Analyses of the recorded data were performed using the statistical package STATA-14. Quantitative and qualitative variables are presented as (mean ± standard deviation) and number (%), respectively. The chi-square and Fisher Exact tests were used to compare qualitative variables between the groups. The Kolmogorov-Smirnov test indicated a normal distribution for the qualitative values related to neurodevelopmental scores. Accordingly, the Independent t-test and Mann-Whitney test were employed to compare the values between groups. Additionally, the Logistic regression test was applied to adjust for the effects of confounders. The first type of error (α) and power of the study (1-β) were set at 0.05 and 80%, respectively.
4. Results
Sixty patients, 30 in each group of congenital or acquired hypothyroidism, were included in the study. The mean levothyroxine administration in the congenital and acquired hypothyroidism groups was 27.28 µg/day and 30.59 µg/day, respectively. Table 1 presents the demographic and clinical data for both groups. The timing of hypothyroidism diagnosis (P = 0.0001) and treatment initiation (P = 0.0001) in the congenital group were significantly earlier than in the acquired group.
| Status and Groups | Gender | Age (mo) | Diagnosis Age | Type of Delivery; NVD/(C.S) | Treatment Onset Age | Maternal Hypothyroidism | |
|---|---|---|---|---|---|---|---|
| Female | Male | ||||||
| Congenital hypothyroidism | 13 (43.3) | 17 (56.7) | 47 ± 8.6 | 18.67 ± 15.21 (d) | 22.97 ± 16.50 (day) | 9 (30) | |
| Acquired hypothyroidism | 16 (53.3) | 14 (46.7) | 47 ± 8.1 | 17.13 ± 15.30 (mo) | 17.16 ± 15.30 (mo) | 16 (53.3) | |
| P-value | 0.606 b | 0.110 c | 0.0001 d | 0.500 b | 0.0001 d | 0.115 b | |
Abbreviations: NVD, normal vaginal delivery; C/S, cesarean section.
a Values are expressed as No. (%), mean ± SD or unless otherwise indicated.
b Fisher Exact test.
c Independent t-test.
d Mann Whitney test.
Final ASQ scores related to neurodevelopmental domains are shown in Table 2. According to ASQ scores and cut-off points, the frequency of abnormal neurodevelopmental status across all domains communication, gross motor, fine motor, problem-solving, and personal-social behavior was higher in the congenital group than in the acquired group; however, the differences between the groups were not significant. The results also revealed that delays in problem-solving and fine motor skills were the most frequent complications in both groups (Table 3).
| Variables | Communication | Gross Motor | Fine Motor | Problem Solving | Personal-Social |
|---|---|---|---|---|---|
| Congenital hypothyroidism | 50.00 ± 13.13 | 50.5 ± 13.54 | 45.33 ± 16.76 | 46.5 ± 14.27 | 50.83 ± 13.27 |
| Acquired hypothyroidism | 53.33 ± 9.68 | 53.83 ± 12.08 | 47.5 ± 15.19 | 50.83 ± 12.04 | 52.67 ± 10.65 |
Abbreviation: ASQ, Ages and Stages Questionnaire.
a Values are expressed as mean ± SD.
| Groups | Abnormal Communication | Abnormal Gross Motor | Abnormal Fine Motor | Abnormal Problem-Solving | Abnormal Personal-Social |
|---|---|---|---|---|---|
| Congenital hypothyroidism | 5 (16.7) | 5 (16.7) | 6 (20) | 7 (23.3) | 5 (16.7) |
| Acquired hypothyroidism | 3 (10) | 3 (10) | 4 (13.3) | 4 (13.3) | 2 (6.7) |
| P-value b | 0.452 | 0.452 | 0.491 | 0.322 | 0.242 |
a Values are expressed as No. (%).
b Fisher’s Exact test.
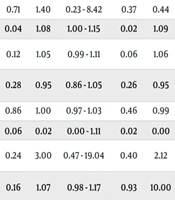
The data analysis revealed that in the congenital hypothyroidism group, the age of diagnosis significantly influenced delays in communication (P = 0.03), gross motor (P = 0.03), fine motor (P = 0.04), and problem-solving (P = 0.02) skills. For each additional day in the age of diagnosis, the risk of abnormalities in these domains increased by 1.10, 2, 1.08, and 1.09 times, respectively. Additionally, low levels of free T4 were significantly associated with abnormalities in communication (P = 0.04), gross motor (P = 0.04), and problem-solving (P = 0.02) skills. Maternal hypothyroidism during pregnancy was also significantly linked to abnormal personal-social behavior (P = 0.02). In children with congenital hypothyroidism, neurodevelopmental status showed no association with gender, age of treatment onset, TSH level, or initial drug dosage (P > 0.05) (Table 4). Moreover, the neurodevelopmental status of children with acquired hypothyroidism was not significantly associated with any demographic or clinical parameters (P > 0.05) (Table 5).
| Areas and Variables | Communication | Gross Motor | Fine Motor | Problem Solving | Personal-Social Behavior | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-Value a | B b | 95% CI c (Lower-Upper) | P-Value a | B b | 95% CI c (Lower-Upper) | P-Value a | B b | 95% CI c (Lower-Upper) | P-Value a | B b | 95% CI c (Lower-Upper) | P-Value a | B b | 95% CI c (Lower-Upper) | |
| Gender | 0.87 | 0.85 | 0.12 - 6.00 | 0.87 | 0.85 | 0.12 - 6.00 | 0.71 | 1.40 | 0.23 - 8.42 | 0.37 | 0.44 | 0.07 - 2.73 | 0.87 | 0.85 | 0.12 - 6.00 |
| Age of diagnosis | 0.03 | 1.10 | 1.01 - 1.20 | 0.03 | 2.00 | 1.01 - 1.20 | 0.04 | 1.08 | 1.00 - 1.15 | 0.02 | 1.09 | 1.01 - 1.17 | 0.14 | 1.05 | 0.98 - 1.12 |
| Age of treatment onset | 0.07 | 1.06 | 0.99 - 1.14 | 0.07 | 1.06 | 0.99 - 1.14 | 0.12 | 1.05 | 0.99 - 1.11 | 0.06 | 1.06 | 10.00 - 1.13 | 0.15 | 1.04 | 0.98 - 1.11 |
| Infancy drug dosage | 0.13 | 0.90 | 0.79 - 1.03 | 0.13 | 0.90 | 0.79 - 1.03 | 0.28 | 0.95 | 0.86 - 1.05 | 0.26 | 0.95 | 0.86 - 1.04 | 0.38 | 0.95 | 0.86 - 1.06 |
| Serum TSH | 0.88 | 1.00 | 0.96 - 1.03 | 0.88 | 1.00 | 0.96 - 1.03 | 0.86 | 1.00 | 0.97 - 1.03 | 0.46 | 0.99 | 0.95 - 1.02 | 0.32 | 0.97 | 0.92- 1.03 |
| Free T4 | 0.04 | 0.00 | 0.00 - 0.86 | 0.04 | 0.00 | 0.00 - 0.86 | 0.06 | 0.02 | 0.00 - 1.11 | 0.02 | 0.00 | 0.00 - 0.46 | 0.05 | 0.01 | 0.00 - 1.09 |
| Maternal hypothyroidism | 0.60 | 1.71 | 0.23 - 12.55 | 0.60 | 1.71 | 0.23 -12.55 | 0.24 | 3.00 | 0.47 - 19.04 | 0.40 | 2.12 | 0.36 - 12.38 | 0.02 | 16.00 | 0.14 -176.45 |
| Maternal drug dosage | 0.42 | 1.03 | 0.95 - 1.12 | 0.42 | 1.03 | 0.95 - 1.12 | 0.16 | 1.07 | 0.98 - 1.17 | 0.93 | 10.00 | 0.93 - 1.06 | 0.68 | 0.99 | 0.93 - 1.05 |
Abbreviations: TSH, thyroid-stimulating hormone; T4, Thyroxine.
a Logistic regression.
b Coefficient.
c %95 Confidence interval for B.
| Areas and Variables | Communication | Gross Motor | Fine Motor | Problem Solving | Personal-Social Behavior | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P-Value a | B b | 95% CI c (Lower-Upper) | P-Value a | B b | 95% CI c (Lower-Upper) | P-Value a | B b | 95% CI c (Lower-Upper) | P-Value a | B b | 95% CI c (Lower-Upper) | P-Value a | B b | 95% CI c | |
| Gender | 0.48 | 0.40 | 0.03 - 4.96 | 0.48 | 0.40 | 0.03 - 4.96 | 0.89 | 0.86 | 0.10 - 7.04 | 0.89 | 0.86 | 0.10 - 7.04 | 0.92 | 0.87 | 0.05 - 15.28 |
| Age of diagnosis | 0.69 | 1.00 | 1.00 - 1.00 | 0.68 | 1.00 | 1.00 - 1.00 | 0.96 | 1.00 | 1.00 - 1.00 | 0.96 | 1.00 | 1.00 - 1.00 | 0.56 | 1.00 | 0.99 - 1.00 |
| Age of treatment onset | 0.69 | 1.00 | 1.00 - 1.00 | 0.68 | 1.00 | 1.00 - 1.00 | 0.96 | 1.00 | 1.00 - 1.00 | 0.96 | 1.00 | 1.00 - 1.00 | 0.56 | 1.00 | 0.99 - 1.00 |
| Infancy drug dosage | 0.87 | 0.99 | 0.92 - 1.07 | 0.54 | 0.97 | 0.90 - 1.06 | 0.62 | 1.01 | 0.95 - 1.08 | 0.62 | 1.01 | 0.95 - 1.08 | 0.55 | 1.02 | 0.95 - 1.11 |
| Heel TSH | 0.24 | 1.17 | 0.90 - 1.52 | 0.53 | 0.90 | 0.64 - 1.26 | 0.38 | 1.11 | 0.88 - 1.40 | 0.38 | 1.11 | 0.88 - 1.40 | 0.12 | 1.31 | 0.93 - 1.86 |
| Serum TSH | 0.77 | 1.84 | 0.03 - 103.22 | 0.77 | 1.84 | 0.03 - 3.22 | 0.60 | 2.62 | 0.07 - 93.29 | 0.60 | 2.62 | 0.07 - 93.29 | 0.79 | 0.51 | 0.00 - 77.10 |
| Free T4 | 0.63 | 1.86 | 0.15 - 23.00 | 0.63 | 1.86 | 0.15 - 23.00 | 0.89 | 0.86 | 0.10 - 7.04 | 0.89 | 0.86 | 0.10 - 7.04 | 0.92 | 0.87 | 0.05 - 15.28 |
| Maternal hypothyroidism | 0.74 | 1.01 | 0.95 - 1.08 | 0.74 | 1.01 | 0.95 - 1.08 | 0.74 | 1.01 | 0.95 - 1.08 | 0.74 | 1.01 | 0.95 - 1.08 | 1.00 | 1.93 | 0.00 - 15.28 |
| Maternal drug dosage | 0.48 | 0.40 | 0.03 - 4.96 | 0.48 | 0.40 | 0.03 - 4.96 | 0.89 | 0.86 | 0.10 - 7.04 | 0.89 | 0.86 | 0.10 - 7.04 | 0.92 | 0.87 | 0.05 - 15.00 |
Abbreviations: TSH, thyroid-stimulating hormone; T4, Thyroxine.
a Logistic Regression.
b Coefficient.
c %95 Confidence Interval for B.
5. Discussion
While previous studies have revealed neurodevelopmental disorders among patients with congenital hypothyroidism, there is limited research regarding neurodevelopmental outcomes in children with acquired hypothyroidism. The present study's results indicated that, based on ASQ scores, the frequency of neurodevelopmental abnormalities was higher in the congenital group compared to the acquired group. However, neurodevelopmental abnormalities in the acquired group were also notable, and no significant difference was observed between the two groups. This finding may emphasize the potential adverse neurodevelopmental outcomes following acquired hypothyroidism, which requires further attention and preventive interventions. Unfortunately, we could not compare our findings with other investigations, as, to our knowledge, no studies have directly compared neurodevelopmental delays between these two groups.
The present study also showed that age at diagnosis and lower T4 levels were significant factors influencing communication, motor, and problem-solving skills in the congenital hypothyroidism group. According to our findings, a delay in diagnosis by even one day could increase the risk of abnormalities in various neurodevelopmental domains by 1.08 to 2 times. Consistent with our results, Razavi et al. (5) investigated the neurodevelopmental status of 78 children with congenital hypothyroidism using ASQ scores. They found that the average age of diagnosis and treatment was 25.65 days in patients with neurodevelopmental impairments and 17.99 days in those without delays. Gross motor delay was the most common disorder and was significantly associated with the age of hypothyroidism diagnosis, initial levothyroxine dosage, and age at treatment initiation. Simic et al. (16) measured a range of higher-order visuocognitive abilities in 19 children and adolescents with congenital hypothyroidism, comparing them with 19 age- and sex-matched typically developing peers using a novel self-report measure of direction sense. Individuals with congenital hypothyroidism exhibited selective visuocognitive weaknesses, some of which were related to early and concurrent TSH levels. Komur et al. (6) also compared the neurological development of 41 patients aged 6 - 42 months with congenital hypothyroidism and 39 healthy controls of the same age group using the Bayley-3 test. Cognitive, language, and global motor scores, in addition to receptive communication, expressive communication, fine motor, and gross motor sub-scores in patients, were significantly lower than those in the control group.
In contrast to our findings, Trumpff et al. (9) assessed the impact of neonatal TSH on psychomotor neurodevelopment during the preschool period. A total of 284 children aged 4 - 6 years were included in their study, and no significant association was found between TSH levels and total motor scores. Differences in inclusion criteria (TSH < 15 vs. confirmed hypothyroidism) and neurodevelopmental assessment tools (Charlop-Atwell Scale vs. ASQ) may explain the contrasting results between Trumpff’s study and others.
According to the results of the present study, maternal hypothyroidism was associated with abnormalities in personal-social behavior. Consistent with our findings, previous studies have identified associations between maternal thyroid hormone insufficiency and neurodevelopmental outcomes in children. Cognitive deficits, attention deficit hyperactivity disorder (ADHD), and autism spectrum disorder (ASD) have been reported in children of mothers with prenatal hypothyroidism (17-19).
It should be noted that the present study had several limitations. The sample size was small, and several potentially influencing factors, such as nutritional status, social intelligence, and parents' education, were not assessed between the groups, which may be other limitations. Long-term follow-up may also provide more informative data. Comparing the congenital and acquired groups with healthy children, especially using larger-scale groups, may be necessary to investigate this concern.
5.1. Conclusions
Although the frequency of neurodevelopmental abnormality in the congenital hypothyroidism group was higher than in the acquired group, no significant difference was observed between the groups. The lack of a significant difference in neurodevelopmental abnormality between the acquired and congenital groups may indicate a similar risk for both. Accordingly, it seems that further considerations, early diagnosis, and appropriate interventions are necessary to prevent adverse neurodevelopmental outcomes following congenital or acquired hypothyroidism. Age at diagnosis, lower T4 levels, and maternal hypothyroidism were significant factors influencing neurodevelopmental status in the congenital group. Further investigations with larger sample sizes are needed to confirm these results.