1. Background
SARS-CoV-2 is a highly transmissible virus that can cause pulmonary and systemic inflammation in patients, known as coronavirus Disease 2019 (COVID-19). The global COVID-19 pandemic has highlighted the intricate interplay between the virus and host immune reactions (1, 2). The proper functioning of the human immune system depends on several factors, such as age, current health issues, and nutritional status. As mentioned in the literature, ensuring an adequate intake of micronutrients such as vitamins and trace elements is important for maintaining overall health and resilience against infectious diseases. In fact, these essential nutrients play a crucial role in regulating the body's immune system and combating the effects of viral illnesses, including COVID-19 (3, 4).
According to one study, the daily administration of vitamin D has a significant impact on reducing damage to alveolar cells in patients with acute respiratory distress syndrome (ARDS) from viral and bacterial infections. Moreover, vitamin D can increase the production of glutathione by enhancing the expression of glutathione reductase and glutamate-cysteine ligase enzymes, and it is recommended for the treatment of COVID-19 infection (5, 6). Another survey conducted in Iran demonstrated that, despite the effects of vitamin D on the cytokine response, supplemental intake of this vitamin can reduce the severe immune response against COVID-19 infection (7). Consistent with this data, the serum levels of vitamin D in the group of Iranian patients with COVID-19 were found to be significantly lower than those observed in the healthy group (8).
Vitamin C is an important micronutrient in the body known for its anti-inflammatory properties and its role in removing free radicals in humans. Moreover, this micronutrient can improve the production of the cortisol hormone, which enhances immunity against different pathogens. Several studies have indicated a synergistic effect on the immune system from vitamin C and a role in the deterioration of mucosal epithelial cells when the levels of this micronutrient are low, resulting in increased susceptibility to COVID-19 (9, 10). Zinc is a trace mineral that is essential for the proper functioning of the immune system and plays a critical role in various stages of tissue growth, such as metabolism, differentiation, and cellular synthesis. Deficiency in this micronutrient has been associated with an increased risk of inflammatory disorders and a higher incidence of microbial pneumonia (11). A study has indicated that sufficient levels of zinc could enhance the host’s resistance against COVID-19 infection (12).
Although some studies have suggested that low levels of zinc, vitamin D, and vitamin C are associated with more severe illness and poorer outcomes in COVID-19 patients, other studies have failed to find a significant association between micronutrient levels and clinical outcomes in COVID-19 patients, leading to conflicting conclusions (13). In light of this uncertainty, it is clear that further research is needed to elucidate the relationship between these micronutrients and clinical outcomes in COVID-19 patients.
2. Objectives
We aimed to compare the serum levels of vitamins D and C, as well as zinc, between healthy children and children with COVID-19 infection, and to investigate deficiencies in patients with mild and severe clinical symptoms.
3. Methods
This case-control study was conducted at Hazrate-Rasool University Hospital in Tehran, Iran, which served as a regional tertiary COVID-19 center with 550 active beds during the pandemic. Control subjects were recruited from children who were referred for elective surgical operations, while cases were children with COVID-19 infection. To analyze infection severity and clinical conditions of patients based on micronutrient levels in their serum, case subjects were divided into two groups: (1) outpatients with mild infection (exhibiting nonspecific symptoms such as cough, fever < 38.5°C, and muscle pain) and (2) hospitalized patients with severe infection (exhibiting symptoms such as hypoxemia with SPO2 < 90%, severe respiratory distress, and pulmonary involvement greater than 50%, etc.) (14).
The sample size was calculated formula below based on serum vitamin D levels estimated from the study by Rastegar et al. (15). This calculation assumed a maximum Type 1 error rate (α) of 0.05, a Type 2 error rate (β) of 0.2, vitamin D standard deviations in the case and control groups (δ) of 4.13 and 4.9, respectively, and average vitamin D levels in cases and controls (µ) of 30.3 and 33.9, respectively. Consequently, the sample size was set at 25 for each group, and eligible subjects were recruited consecutively for the study.
The inclusion criteria for the study were defined as being aged ≤ 15 years, having negative PCR tests for SARS-CoV-2 in the control group, and positive PCR tests in the case group. Subjects with underlying comorbidities such as kidney and liver disease, those with malnutrition, and those using steroids and/or any food supplement containing vitamin C, D, or zinc were excluded from the study. This study was approved by the ethics committee of Iran University of Medical Sciences (IR.IUMS.FMD.REC.1402.071), and participants signed a consent form. Demographic information and clinical data of participants were documented through physical examination or via their medical records.
Blood specimens (5 cc) were obtained from participants and sent to the research laboratory at the Institute of Immunology and Infectious Diseases for assessment of the micronutrient levels (vitamin D, vitamin C, and zinc). Initially, the serum was isolated from blood samples according to previously described procedures, transferred to new tubes, and kept at -20°C until further analysis (16). The measurement of serum levels of vitamin D and vitamin C was performed using commercial kits (Padtan Gostar Co, Iran) through the enzyme-linked immunosorbent assay (ELISA). Serum levels less than 31 ng/mL and ≤ 5 μg/mL were considered as vitamin D and vitamin C deficiencies, respectively. Additionally, serum concentrations of zinc were determined using commercial kits (Kiazist Co, Iran) through a direct colorimetric assay. The reference cut-off for zinc concentration was set at < 10.7 µmol/L.
3.1. Statistical Analysis
For continuous variables, data were presented as means and standard deviations (SD) and compared using the t-test. For categorical variables, the chi-square test was used to detect differences between the two groups. The data were analyzed using MedCalc Statistical Software version 15.8 (MedCalc Software bvba, Ostend, Belgium) and GraphPad Prism software, version 5.0 (GraphPad Software). A P-value of less than 0.05 was considered significant (17).
4. Results
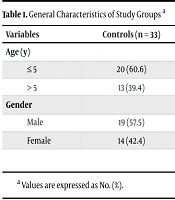
A total of 96 children were recruited for the investigation, of which 11 children were excluded due to dissatisfaction with involvement, resulting in 85 children being surveyed (33 healthy children in the control group, and 25 and 27 children with mild and severe clinical symptoms in the case groups, respectively). The main symptoms documented in children with mild symptoms included fever, cough, and diarrhea. Additionally, the most common clinical symptoms in patients with severe infection were gastrointestinal problems, tachycardia, and a high degree of fever. The general characteristics of the study population are displayed in Table 1.
| Variables | Controls (n = 33) | Cases with Mild Symptoms (n = 25) | Cases with Severe Symptoms (n = 27) |
|---|---|---|---|
| Age (y) | |||
| ≤ 5 | 20 (60.6) | 17 (68.0) | 18 (66.6) |
| > 5 | 13 (39.4) | 8 (32.0) | 9 (33.4) |
| Gender | |||
| Male | 19 (57.5) | 10 (40.0) | 14 (51.8) |
| Female | 14 (42.4) | 15 (60.0) | 13 (48.2) |
a Values are expressed as No. (%).
Generally, the serum levels of vitamin D between the studied groups were almost similar, and no significant differences were observed in the statistical analysis. Moreover, although the mean serum levels of vitamin C were higher in the control group (12.5 ng/mL) than in the patient groups (11.1 ng/mL and 10.7 ng/mL, respectively), these differences were not statistically significant. Conversely, the serum zinc levels were significantly lower in patients with severe clinical symptoms compared to the controls (P < 0.001). Table 2 displays the comparison of the mean values of micronutrients between the studied groups in this study.
a Vaues are expressed as mean ± SD.
b Compared to the controls.
c Significant.
Regarding the impact of vitamin D, C, and zinc deficiencies on the severity of infection, no statistically significant difference was observed in the frequency of children with micronutrient deficiencies between the two groups of patients with mild and severe clinical conditions of COVID-19. Table 3 shows the frequencies of patients with micronutrient deficiencies in the case groups. Regarding the impact of vitamin D, C, and zinc deficiencies on the severity of infection, no statistically significant difference was observed in the frequency of children with micronutrient deficiencies between the two groups of patients with mild and severe clinical conditions of COVID-19. Table 3 shows the frequencies of patients with micronutrient deficiencies in the case groups.
a ≤ 31 ng/mL.
b ≤ 5 μg/mL.
c ≤ 10.7 µmol/L.
5. Discussion
The spread of the COVID-19 pandemic worldwide has been associated with a wide range of intensities and severities of clinical symptoms, which has become the subject of many studies aimed at finding the cause of this issue. One area of investigation is determining the body's need for vitamins and essential elements such as vitamin D, vitamin C, and zinc (18).
Overall, several studies have indicated that high levels of vitamin D might be supportive in COVID-19 infections by regulating the immune response and its reaction to infection (19). However, based on our findings in the current study, the mean serum levels of vitamin D in children with mild and severe clinical conditions of COVID-19 were similar to those found in healthy control children. In other words, there were sufficient levels of vitamin D in the serum of most children patients when infected or hospitalized due to COVID-19. This result aligns with findings from a survey reported in Spain, where researchers found that vitamin D had no effect on reducing mortality or the severity of clinical symptoms in patients with COVID-19 (20). Additionally, in a study from the UK, Hastie et al. reported no associations between vitamin D levels and COVID-19 (21).
Conversely, Karimian et al. reported an association between vitamin D levels in children and the severity of clinical signs, gastrointestinal problems, and the extent of involvement in patients. They also believed that this vitamin should be considered a critical issue in the management of patients (7). Another study from Turkey indicated a significant difference between the serum levels of vitamin D in COVID-19 patients compared to children in the control group. Furthermore, the lack of vitamin D in children was reported to be negatively related to the appearance and incidence of fever, with fever symptoms recorded more intensely in this category of children (with vitamin D deficiency) (22). Variation in genetic backgrounds among the studied populations can be considered one reason for the differing results between studies. Moreover, differences in experimental methodologies and the sensitivity of the measurement tests may contribute to the inconsistencies in results. Overall, findings from these reports are still controversial, and further research is needed to verify potential associations in patients.
We found that COVID-19 patients had lower levels of vitamin C compared with healthy, non-infected children (mean, 12.5 vs. 10.9 ng/mL). Furthermore, severe COVID-19 children had lower vitamin C concentrations than mild patients (mean, 10.7 vs. 11.1 ng/mL). However, most subjects in both control and case groups had sufficient levels of vitamin C in their serum, and no significant difference was observed between the patients with vitamin C deficiency based on disease severity. Consistently, Ramezaninejad et al., in a retrospective cross-sectional study, evaluated the effectiveness of vitamin C on the clinical outcomes of patients with COVID-19 and found no remarkable differences between those who received this micronutrient and those who did not, in terms of survival and the need for mechanical ventilation (23). Also, Beran et al. conducted a meta-analysis including 5,633 patients and found no significant relationship between vitamin C intake and mortality rates in patients (24). In addition, Gao et al. in China pointed out that vitamin C did not play a role in reducing the mortality of patients and was not associated with a reduction in infection (25).
Our study also showed that the serum zinc levels in severe COVID-19 cases (10.6 µmol/L) were statistically lower than those in healthy controls (13.7 µmol/L). Moreover, nine (33.4%) severe cases had zinc deficiency, while only four (16.0%) mild patients had zinc deficiency. The effectiveness of zinc on the clinical condition of patients was investigated in an Iranian study, which reported no effect on reducing adverse outcomes associated with this micronutrient (26). In 2021, Yao et al. conducted a retrospective cohort study investigating zinc concentrations among hospitalized patients with COVID-19 infection and reported that this micronutrient had no effect on decreasing the death rate of patients (27). Additionally, results from a randomized controlled trial showed that zinc supplementation did not influence mortality rates among patients with COVID-19 infection (28). However, zinc deficiency in people's diets has been reported in many countries, with approximately 25% of the world's population facing the risk of zinc deficiency (29).
5.1. Limitations
The current study is among the few investigations in Iran conducted on children with COVID-19. However, some limitations should be mentioned before interpreting the data. First, the concentration of serum micronutrients was not adjusted to account for age status and physiological variations (male, female) among children. Secondly, subjects were recruited from a single center, and the findings may not be generalizable to other regions.
5.2. Conclusions
In conclusion, the comparison of serum zinc levels between healthy children and children with severe COVID-19 infection in this study was significant. However, this study did not find a relationship between the lack of micronutrients and the severity of the disease. Ultimately, well-designed, large-scale studies are necessary to provide more robust evidence and address the limitations of previous research. Additionally, the potential for randomized controlled trials to investigate the impact of micronutrient supplementation on COVID-19 outcomes should be explored.