1. Background
Ventilator-associated pneumonia (VAP) is among the most common nosocomial infections in intensive care units (ICUs) (1), with a prevalence ranging from 5% to 40% (2). Patients admitted to the ICU face high mortality risks not only due to their critical conditions but also from secondary complications, such as hospital-acquired infections (3). Ventilator-associated pneumonia is associated with prolonged ICU stays, increased healthcare costs, and elevated morbidity and mortality rates (4).
The rise in bacterial resistance, largely due to the increased use of broad-spectrum antibiotics and inadequate infection control measures, has become a pressing global issue in medicine (5). Multidrug-resistant gram-negative bacterial (MDR-GNB) infections are increasingly prevalent in ICU patients with VAP, involving pathogens such as Pseudomonas aeruginosa, extended-spectrum beta-lactamase-producing Klebsiella, and Acinetobacter baumannii (6, 7). The primary pathogens causing VAP in ICU patients are Acinetobacter baumannii, Pseudomonas aeruginosa, and Klebsiellapneumoniae (8, 9). Resistance to β-lactams (including carbapenems), aminoglycosides, and fluoroquinolones has been increasingly observed in these MDR pathogens (10). The virulence of GNB pathogens has significantly restricted effective treatment options (11, 12).
Polymyxins, introduced into clinical use in the late 1950s, include colistin sulfate, polymyxin B, and colistimethate sodium, and studies have demonstrated their efficacy against MDR-GNB infections (13). Colistin exhibits rapid, concentration-dependent bactericidal activity against gram-negative pathogens, including P. aeruginosa, A. baumannii, and K. pneumoniae (14). Colistin is widely considered for its potent bactericidal effect, low resistance incidence, and excellent activity against gram-negative bacilli (15).
Recent research suggests that nebulized antibiotics can be effective as an adjunctive treatment alongside intravenous antibiotics for treatment-resistant strains (13). While intravenous colistin is often associated with side effects, primarily nephrotoxicity and neurotoxicity (16, 17), the inhaled form is preferred for its limited side effects (15).
Current studies have examined the efficacy of intravenous colistin as an adjunctive or alternative treatment, but limited research addresses the efficacy and safety of inhaled colistin. Furthermore, information on the safety and efficacy of inhaled colistin in pediatric patients remains sparse (18).
2. Objectives
This study was thus conducted to assess the efficacy of adjunctive inhaled colistin in critically ill children with multidrug-resistant ventilator-associated pneumonia (MDR-VAP).
3. Methods
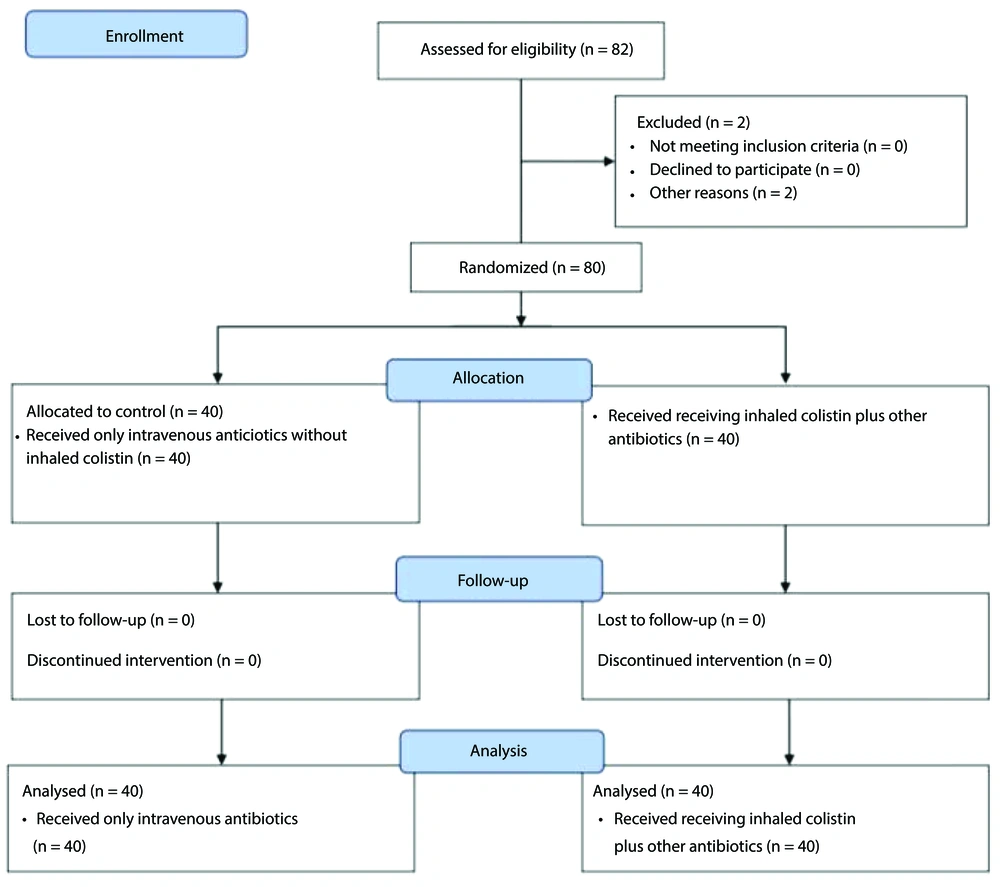
In this prospective cohort study, eighty critically ill patients aged 2 to 18 years with MDR-VAP were admitted to the PICU at Mofid Children's Hospital in Tehran between 2019 and the end of 2022.
The inclusion criteria were as follows: Patients aged 2 to 18 years with a positive culture for MDR-GNB, diagnosed with VAP, and progressive pulmonary infiltrates based on chest radiograph reports. Exclusion criteria included patients with positive cultures for MDR-GNB who had been treated with nebulized colistin sulfate for less than 3 days, patients who died within 48 hours, those with renal failure, lung cancer patients with obstructive pneumonia, patients sensitive to colistin, and patients with incomplete files.
For the diagnosis of VAP, criteria included fever (T > 38.5°C) or hypothermia (T < 36°C), leukocytosis (WBC > 12,000/mm³) or leukopenia (WBC < 4,000/mm³), and the development or progression of pulmonary infiltrates on chest X-ray. The validation of VAP involved testing bronchial secretions or bronchoalveolar lavage (BAL) for MDR gram-negative bacteria in a positive culture. The diagnostic threshold for identifying the microorganism’s causing VAP was set at 104 colony-forming units per milliliter in BAL.
Two patients were excluded due to mortality on the second and fourth days of the study, leaving a final analysis sample of 80 patients (Figure 1). Eligible patients were divided into two equal groups: A colistin group (receiving inhaled colistin plus other antibiotics) and a control group (receiving only intravenous antibiotics without inhaled colistin).
Inhaled colistin treatment began as soon as a culture confirmed MDR-GNB infection. The dosing regimen was 3 to 5 mg/kg every 6 hours for a duration of 2 to 3 weeks, maintained for at least two weeks. After extubation, the colistin dose was adjusted to account for a 40% extrapulmonary deposition, based on findings from experimental studies (19).
Pneumonia was diagnosed through computed tomography (CT) imaging by a pulmonologist and radiologist. Data collection involved a structured questionnaire to record patient characteristics, including age, sex, clinical symptoms, laboratory data, clinical outcomes, days under mechanical ventilation, ICU stay duration, and mortality.
To assess the efficacy of adjunctive inhaled colistin in critically ill children with MDR-VAP across the two groups, a sample size of 80 patients was determined based on data from a previous study (5) and calculated using an appropriate statistical formula. The statistical power was set at 80% with a 95% confidence level.
3.1. Statistical Analysis
Statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS) software version 22 (IBM, Chicago, USA). Quantitative variables were expressed as mean ± SD, while qualitative variables were presented as number (percentage). Distribution was evaluated using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Differences were assessed using the t-test or Mann-Whitney U test as appropriate. Chi-square/Fisher’s exact test was applied to compare frequencies across groups. A result was considered statistically significant if the P-value was less than 0.05.
4. Results
The mean age of participants in the control and colistin groups was 21.2 ± 2.9 and 24.9 ± 1.7 months, respectively (P = 0.51). The study included 35 girls and 45 boys, with female participants making up 42.5% of the control group and 45% of the colistin group; this gender distribution was not statistically significant (P = 0.61). Regarding mortality, 26 children (65%) in the control group and 23 children (57.5%) in the colistin group died, with no significant difference in mortality rates between the groups (P = 0.49) (Table 1).
| Variables | Control Group; (n = 40) | Colistin Group; (n = 40) | P-Value b |
|---|---|---|---|
| Age (mo) | 21.2 ± 2.9 | 24.9 ± 1.7 | 0.51 |
| Gender (female) | 17 (42.5) | 18 (45.0) | 0.61 |
| Mortality | 26 (65) | 23 (57.5) | 0.49 |
a Values are expressed as mean ± SD or No. (%).
b Chi-squared test.
White blood cell (WBC) counts for the control and colistin groups were 13,120 ± 7,821.08 and 21,871.79 ± 35,818.61 mm³, respectively (P = 0.31). Neutrophil percentages were 65.29 ± 19.69 in the control group and 68.27 ± 13.84 in the colistin group, also showing no significant difference (P = 0.44). Lymphocyte percentages were 27.78 ± 18.07 and 24.04 ± 14.18 for the control and colistin groups, respectively, with no significant difference (P = 0.31). Hemoglobin levels between the control and colistin groups were similar (9.44 ± 1.70 vs. 9.18 ± 1.89 g/dL, P = 0.51), as were creatinine levels (0.62 ± 0.43 vs. 0.55 ± 0.51, P = 0.52).
There were also no significant differences between the two groups in other laboratory markers, such as erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels (P > 0.05). Further laboratory details are provided in Table 2.
| Variables | Control Group; (n = 40) | Colistin Group; (n = 40) | P-Value b |
|---|---|---|---|
| WBC (mm3) | 13120 ± 7821.08 | 21871.79 ± 35818.61 | 0.31 |
| Neutrophil (%) | 65.29 ± 19.69 | 68.27 ± 13.84 | 0.44 |
| Lymphocyte (%) | 27.78 ± 18.07 | 24.04 ± 14.18 | 0.31 |
| Hb (g/dL) | 9.44 ± 1.70 | 9.18 ± 1.89 | 0.51 |
| Creatinine (mg/dL) | 0.62 ± 0.43 | 0.55 ± 0.51 | 0.52 |
| Albumin (g/dL) | 3.40 ± 0.69 | 3.44 ± 0.50 | 0.78 |
| ESR (mm/hr) | 46.27 ± 35.82 | 48.05 ± 27.65 | 0.85 |
| CRP (mg/dL) | 33.18 ± 22.65 | 41.35 ± 20.0 | 0.12 |
Abbreviations: WBC, white blood cells; Hb, Hemoglobine; ESR, Erythrocyte Sedimentation Rate; CRP, C-reactive protein.
a Values are expressed as mean ± SD.
b independent t-test.
The duration of hospital stay did not differ significantly between the control and colistin groups (29.83 ± 22.35 vs. 34.92 ± 20.03 days, P = 0.29), nor did the days of mechanical ventilation (23.15 ± 21.17 vs. 24.06 ± 17.87 days, P = 0.83). However, fever occurrence was significantly higher in the control group than in the colistin group (7.5% vs. 2.5%, P = 0.04). No neurotoxicity was observed in either group. Nephrotoxicity was reported in 2.5% of the colistin group and 7.5% of the control group, though this difference was not statistically significant (P = 0.305). Tachycardia was observed in 70% of the control group and 80% of the colistin group, with no significant difference between them (P = 0.302) (Table 3).
| Variables | Control Group; (n = 40) | Colistin Group; (n = 40) | P-Value b |
|---|---|---|---|
| Days of hospitalization | 29.83 ± 22.35 | 34.92 ± 20.03 | 0.29 |
| Days of using mechanical ventilation | 23.15 ± 21.17 | 24.06 ± 17.87 | 0.83 |
| Nephrotoxicity | 3 (7.50) | 1 (2.50) | 0.305 |
| Neurotoxicity | 0 | 0 | - |
| Fever | 26 (65) | 17 | 0.04 |
| Tachycardia | 28 (70) | 32 (80) | 0.302 |
| Bronchospasm | 0 | 0 | - |
a Values are expressed as mean ± SD or No. (%).
b independent t-test
5. Discussion
This study aimed to investigate the effect of inhaled colistin in critically ill children with MDR-VAP. Our findings showed no significant difference between the two groups regarding days on mechanical ventilation, length of hospital stay, or mortality rates. Limited studies have evaluated the efficacy of aerosolized colistin in treating VAP (20).
In contrast to our results, Bharathi et al. reported a statistically significant reduction in the duration of mechanical ventilation and hospitalization among VAP patients treated with colistin compared to those receiving normal saline (5, 21). Similarly, Bao et al. found that while inhaled colistin did not significantly impact days of mechanical ventilation or 28-day mortality, it did reduce hospitalization days compared to the control group (17 vs. 23 days, P=0.01) (18). Unlike Bao et al.'s (18) findings, our study showed no difference in hospital stay between the colistin and control groups. Methodological and study design differences likely contribute to these varied outcomes. Additionally, differences in atomization devices and dosing regimens should be considered, as the limited efficacy of nebulizers in delivering aerosols to peripheral lung areas may have impacted our results.
Our study also showed no reduction in mortality in the colistin group, consistent with Bharathi et al., who found no significant difference in mortality between colistin and normal saline groups (5). Feng et al. similarly reported that adjunctive nebulized colistin did not lower the 14-day mortality rate (22). Korbila et al. also observed no significant difference in mortality between colistin and control groups (23). However, Michalopoulos et al. found that adding colistin significantly reduced mortality (24). Notably, multiple studies have indicated that aerosolized colistin does not lower overall mortality (25-27). These findings suggest that this treatment approach may not have a definitive effect on mortality rates. Mortality in critically ill patients with VAP is influenced by several factors beyond infection control, including disease severity and comorbid conditions.
Nephrotoxicity and neurotoxicity are known adverse effects of colistin (28, 29). However, in the study by Feizabadi et al., these side effects were not observed (30). Similarly, in the study by Bao et al., the incidence of nephrotoxicity was 16.1% in the colistin group compared to 9.7% in the control group, with no significant difference between the groups (18). In our study, although nephrotoxicity was more frequent in the control group, this difference was not statistically significant (P = 0.302). Variability in nephrotoxicity across studies could be attributed to differences in colistin dosage, formulation, and type—colistin sulfate, used in earlier studies, is more toxic than the currently used colistimethate sodium.
Almangour et al. reported no neurotoxicity associated with colistin use (31), consistent with our findings, as neurotoxicity was not observed in our study. The discrepancies across studies may stem from differences in colistin dosage, treatment duration, and variations in concurrently administered intravenous antibiotics.
The main limitations of our study include its retrospective, single-center design and the relatively small sample size, which limit the generalizability of clinical outcomes, laboratory findings, and observed side effects. Additionally, the co-administration of other antibiotics with colistin may have influenced the results. Further research is needed to assess the impact of adjunctive inhaled antibiotics on specific patient subgroups and to investigate the potential development of antibiotic resistance.
5.1. Conclusions
We concluded that inhaled colistin did not reduce the duration of hospitalization, the duration of mechanical ventilation use, or the mortality rate. In other words, there was no significant difference in clinical and laboratory outcomes between the inhaled colistin group and the control group in pediatric VAP patients. Further multicenter studies with larger sample sizes are recommended to better elucidate the potential effects of colistin in patients with VAP caused by MDR pathogens.