1. Introduction
Wilms tumor is the leading pediatric renal and abdominal malignancy, and it ranks fourth among all pediatric malignancies, with around 500 new cases reported annually in the United States. It is assumed to result from abnormal renal development, and many genetic alterations underlying the disease occur in genes involved in fetal nephrogenesis.
Ifosfamide is an alkylating agent used to treat various solid tumors in pediatric and adult patients, including Wilms tumor. Depending on the indication, it can be used as a monotherapy or with other chemotherapeutic agents.
Recently, there has been an increase in the use of ifosfamide, carboplatin, and etoposide (ICE) combination therapy in the treatment of refractory or relapsed solid tumors in pediatric patients, with favorable overall response rates exceeding 50% for a variety of recurrent and refractory pediatric solid tumors (1). Despite its efficacy, ifosfamide is associated with a range of side effects, including hemorrhagic cystitis, nephrotoxicity, myelosuppression, and encephalopathy.
Ifosfamide-induced encephalopathy (IIE) is a rare yet serious condition with a spectrum of signs and symptoms ranging from mild lethargy to coma or death. Despite its severity, many aspects of this condition remain poorly understood. Here, we present the course of IIE in a four-year-old girl with Wilms tumor. Our case report provides a detailed account of the onset, progression, and effective management of IIE using methylene blue.
This case is notable for using a methylene blue protocol adapted from another condition, which successfully alleviated the patient’s symptoms. It underscores the challenges of managing IIE in pediatric patients, especially given the lack of standardized treatment guidelines. Additionally, the presence of controversial risk factors in our patient offers new perspectives on their potential role in IIE, calling for further investigation into these factors.
2. Case Presentation
We present the case of a 4-year-old girl diagnosed as a case of right kidney Wilms tumor with multiple lung metastases, classifying it as Stage 4. Following the diagnosis, radical nephrectomy was performed, revealing diffuse anaplasia on histopathology.
The patient commenced chemotherapy post-surgery using the UH-1 (unfavorable histology) protocol. Within three weeks, operational complications led to contralateral kidney ischemia, compounding the loss of function in the remaining kidney and necessitating routine hemodialysis.
Six weeks into the chemotherapy, the patient received radiotherapy for the tumor bed and lungs over six sessions due to an incomplete response to chemotherapy after six weeks, following COG protocols (2).
Based on the patient's bilateral kidney impairment, a modified UH-1 regimen was devised for the patient, excluding carboplatin and adjusting the dosages of other chemotherapeutic agents. Throughout chemotherapy, the patient experienced multiple episodes of permcath infections and febrile neutropenia, requiring antibiotic treatment along with GCSF. A significant limitation encountered was the patient's renal insufficiency, which prevented the use of antibiotics with nephrotoxic features.
After 24 weeks of chemotherapy, there were no tumor remnants or metastases. Relapse, however, was revealed six weeks later by a tumor mass adjacent to the primary site and two subpleural nodules.
Due to the "very high-risk relapse" nature of the tumor, an adjusted ICE protocol was initiated, with plans for surgery and radiation therapy to follow.
The chemotherapy regimen involved ifosfamide at 1800 mg/mm² for three days, accompanied by Mesna in proportion to the dosage of ifosfamide, and etoposide at 100 mg/mm² for three days. Carboplatin was discontinued from the protocol due to the patient's kidney condition.
On the first day of the ICE protocol, ifosfamide was infused over six hours, along with adequate hydration. Six hours after the infusion, the patient developed severe agitation, decreased consciousness, hallucinations, and tachycardia. Notably, the patient did not display a fever, had normal blood pressure, and her pupils were equal in size and reactive to light.
A comprehensive laboratory test was ordered and the test results indicated neutropenia, low platelet count, hypocalcemia, hypomagnesemia, elevated liver enzymes, elevated erythrocye sedimentation rate (ESR) and C-reactive protein (CRP), elevated creatinine, hypoalbuminemia, and metabolic acidosis, which are detailed in Table 1.
| Variables | Values |
|---|---|
| WBC | 2.7 |
| Lymphocyte | 34% |
| RBC | 3.8 |
| PLT | 76 |
| PTT | 31 |
| Sodium | 139 |
| Calcium | 7.8 |
| Phosphorus | 5.8 |
| ALT | 82 |
| ESR | 15 |
| LDH | 756 |
| Direct bilirubin | 0.16 |
| Urea | 62 |
| Albumin | 3.6 |
| Direct COOMBS | Negative |
| U/A | Normal |
| Blood culture | Negative |
| Neutrophil | 45% |
| Hb | 10.9 |
| HCT | 32.7 |
| PT | 12 |
| INR | 1 |
| Potassium | 5.1 |
| Magnesium | 1.83 |
| AST | 63 |
| ALP | 342 |
| CRP | Weakly + |
| Total bilirubin | 0.6 |
| Blood sugar | 109 |
| Cr | 1.88 |
| Total protein | 5 |
| URIC ACID | 5.9 |
| Urine culture | Negative |
| ABG | |
| PH | 7.26 |
| PCO2 | 24.3 |
| HCO3 | 10.3 |
Laboratory Test Results of the Patient
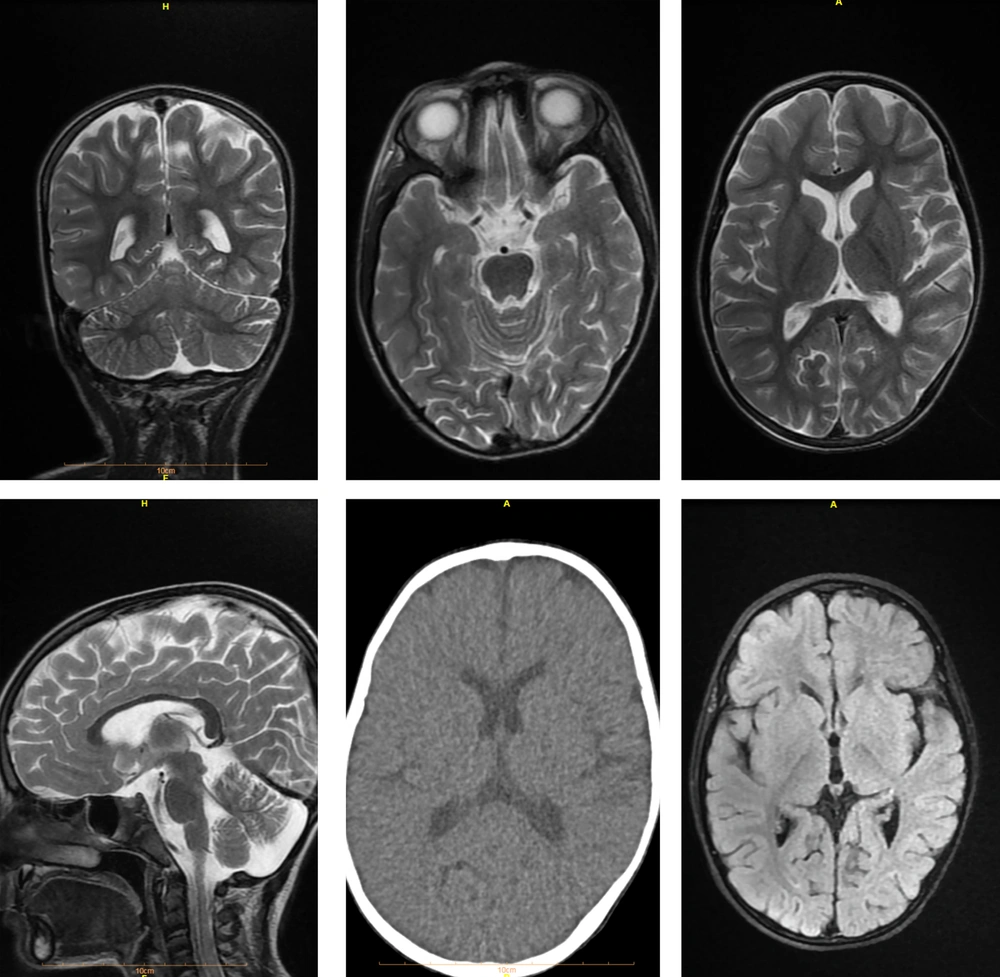
Upon suspicion of encephalopathy, the patient underwent brain imaging, revealing no evidence of bleeding or abnormalities in the white-gray matter, as shown in Figure 1.
Upon suspecting IIE, we withheld ifosfamide, began hydration therapy and serum alkalization, and administered 1 mg/kg of methylene blue intravenously for 30 minutes.
The patient was under continuous medical supervision throughout the treatment. Regular clinical assessments and laboratory tests were conducted to identify and manage potential side effects. Methylene blue administration was well-tolerated, with no adverse effects observed.
Less than 6 hours after MB infusion, the symptoms gradually subsided, and within another 6 hours, all symptoms resolved, confirming the diagnosis of IIE.
As a result, we canceled the second dose of ifosfamide, and the patient exhibited no similar symptoms after that.
3. Discussion
Ifosfamide-induced encephalopathy is documented in 5 - 30% of all patients undergoing ifosfamide treatment, with a lower incidence observed among pediatric patients, occurring in approximately 13% of cases (3).
In most cases, ifosfamide-related neurotoxic effects develop between 12 and 146 hours following the initiation of ifosfamide administration. In our patient, the initial signs and symptoms of IEE appeared twelve hours after initiating the infusion.
If ifosfamide is discontinued, encephalopathy symptoms typically subside within 48 - 72 hours. There are, however, reports of IIE progressing to coma and death, highlighting the critical need for interventions to reverse the situation.
Ifosfamide encephalopathy presents a variety of signs and symptoms. The most common manifestation is confusion, ranging from lethargy to delirium and hallucinations. Less common manifestations include extrapyramidal symptoms, cranial nerve abnormalities, seizures, mutism, dysarthria, and asterixis. Ifosfamide-induced encephalopathy in children can rarely cause developmental milestone loss, progressive brain atrophy, cranial growth cessation, sensory peripheral neuropathy, extrapyramidal toxicity, and subacute CNS degeneration (4).
Despite recent findings linking IIE to EEG patterns including generalized periodic discharges (GPDs) with or without triphasic morphology, interspersed with intermittent background attenuation, and accompanied by characteristic orofacial myoclonus (5), clinical suspicion remains the foundation for diagnosing IIE. Ifosfamide-induced encephalopathy has not been associated with any specific radiologic findings.
According to the National Cancer Institute, IIE can be classified into four grades: Grade I: Dazed or slightly depressive periods; grade II: Extensive sleep or agitation; grade III: Heavy depression, mild hallucinations, or a stuporous condition; grade IV: State of hallucinations or coma.
Based on our patient's classification as experiencing grade III IIE, the treatment with Methylene Blue was initiated.
Several risk factors have been suggested for IIE, including: High doses of ifosfamide, shorter infusion times of ifosfamide, frequency of ifosfamide administration, poor performance status (ECOG 2 to 4), the concomitant use of cisplatin or carboplatin, aprepitant or a CYP3A4 inhibitor, kidney dysfunction (creatinine > 1 mg/dL), hepatic impairment, elevated liver enzymes (ALT > 65 IU/L and AST > 40 IU/L), hypoalbuminemia (especially if < 35 g/L), hyperbilirubinemia (total bilirubin > 3 mg/dL), hypocalcemia, anemia, bulky pelvic disease, sarcoma (compared to lymphoma), and brain metastasis.
However, the consensus on risk factors contributing to the development of IIE is still uncertain. According to a recent review article, only a performance status of 2 to 4, multiple risk factors, renal insufficiency, and hypoalbuminemia appear to be risk factors for the development of IIE in the adult population, while data on other potential risk factors is controversial and limited, and more research is needed to confirm or entirely deny their role as risk factors (6).
There is limited data on potential risk factors for IIE in pediatric patients. According to Ide et al. (7), the only risk factor for pediatric IIE development is the co-administration of ifosfamide with CDDP or CBDCA. Further studies are required to determine the risk factors of IIE in the pediatric population.
In our patient's case, anemia, elevated liver enzymes, hypocalcemia, and kidney dysfunction may have contributed to the development of IIE.
Despite their recognition in adult populations, data confirming their role in pediatric cases remain inconclusive. In this case, the presence of these risk factors in a child with subsequent IIE development highlights the need for further investigation into these risk factors in pediatric populations.
The mechanisms underlying IIE remain largely unknown. The most widely accepted hypothesis is that IIE is primarily attributed to the neurotoxic metabolite Chloroacetaldehyde (CAA), produced during the hepatic activation of ifosfamide by cytochrome P450. Chloroacetaldehyde crosses the blood-brain barrier and induces neurotoxicity by inhibiting the respiratory chain. This leads to the accumulation of nicotinamide adenine dinucleotide hydrogen (NADH) and prevents the dehydrogenation of aldehyde, which results in the formation of CAA (8).
The treatment of IIE focuses on reducing excess electrons and restoring the mitochondrial respiratory chain, which increases CAA metabolism. Methylene blue has traditionally been used for this purpose. The first use of methylene blue in treating IIE was based on the observation of increased urinary excretion of Glutaric acid and Sarcosine, similar to Glutaric Aciduria type II, which was successfully managed with methylene blue. Methylene blue is used as a treatment for IIE due to its role as an alternative electron acceptor, replacing the flavoproteins and restoring the activity of Glutaryl-CoA Dehydrogenase. This helps prevent the accumulation of neurotoxic metabolites. Methylene blue also aids in the oxidation of accumulated NADH, repairs hepatic gluconeogenesis, and inhibits amine oxidase activity, thereby preventing CAA formation and transformation (8).
However, as most of the evidence supporting the use of methylene blue in treating IIE comes from case reports, further studies are essential to determine its effectiveness.
Besides methylene blue, albumin and thiamine are also used to treat IIE; however, methylene blue is more commonly utilized.
Existing data on the use of methylene blue as a prophylactic measure for IIE is limited and shows variable results. Despite some reports suggesting that methylene blue prophylaxis prevents IIE and reduces its severity when it occurs (9), others state that methylene blue, thiamine, or albumin do not significantly reduce the risk of IIE development.
Prophylaxis may allow previously affected individuals to resume treatment and complete chemotherapy post-recovery from neurotoxic effects, and enable high-risk patients to start treatment. Thus, further studies are imperative to determine the effectiveness of methylene blue in preventing IIE in adult and pediatric populations.
In most cases of IIE, symptoms typically resolve within 2 - 4 days after ifosfamide discontinuation. Consequently, the circumstances warranting methylene blue administration to patients experiencing IIE remain unclear. Methylene blue may, however, be considered for high-risk patients with grade III or IV neurotoxicity, as irreversible neurological damage and death can develop (10).
Based on our patient's classification as experiencing grade III IIE, we initiated treatment with methylene blue.
The data on methylene blue dosage recommendation for prophylaxis and treatment is limited and primarily based on Pelgrims et al.'s (11) suggestion of administering 50 mg intravenously six times daily for treatment and 50 mg taken orally or intravenously four times daily for prophylaxis. It is recommended to continue using methylene blue until neurotoxic symptoms are alleviated in the treatment phase. Notably, intravenous administration results in a significantly higher concentration compared to the oral route, which becomes crucial, especially considering the CNS-targeted action of some toxic ifosfamide metabolites.
There is limited experience in managing IIE in children. In our patient care, the lack of valid guidelines for methylene blue dosage recommendations for treating IIE in pediatric patients presented a significant challenge. Following the Euro Ewing 2012 protocol (12), we administered methylene blue intravenously at a dose of 1 mg/kg over 30 minutes, and the symptoms gradually subsided within 12 hours. This successful application of Methylene Blue, adapted from protocols for other conditions, underscores the practical utility of this regimen, specifically for managing IIE in pediatric patients. Our case report highlights the effectiveness of this treatment and adds to the limited data on managing IIE in children, stressing the need for standardized protocols in this area.
There have been no reports of interactions between methylene blue and ifosfamide pharmacokinetics. However, it may cause a variety of adverse effects, including bluish-green discoloration of urine, limb pain following IV administration, dizziness, confusion, and headaches.
3.1. Conclusions
In conclusion, ifosfamide, while integral to diverse treatment protocols, can present a notable adverse effect called IIE. This condition, though often reversible, can progress to severe manifestations, including coma and death. Methylene blue shows significant potential in preventing and reversing the neurotoxic effects of ifosfamide.
We strongly advise pediatric oncologists and hematologists to proactively consider the potential occurrence of IIE and assess patients for possible risk factors associated with IIE before commencing ifosfamide therapy. It is recommended to consider methylene blue as a prophylactic measure for patients with significant risk factors and as a therapeutic agent in cases where IIE manifests. This is especially crucial for patients with a history of its occurrence or those presenting various risk factors predisposing them to its development.
Given the lack of standardized guidelines for managing IIE, particularly in pediatric patients, we suggest the development of a risk-based guideline to improve the management of this condition. Such a guideline would involve a comprehensive assessment of risk factors before initiating Ifosfamide therapy. Based on a risk score, different preventive or monitoring strategies could be applied:
Low risk score: Ifosfamide therapy can proceed as planned.
High risk score: Preventive administration of methylene blue should be considered.
Medium risk score: Patients should undergo close monitoring for early signs and symptoms of IIE, with prompt intervention if neurotoxicity develops.
Developing such a guideline could help standardize preventive strategies and improve patient outcomes.
Finally, due to limited reports on the use of methylene blue for IIE in children, further research is imperative to confirm the efficacy of methylene blue in preventing and reversing IIE and to establish the optimal dosage for both preventive and therapeutic purposes.
3.2. Takeaway Lessons
Assess risk factors: Evaluate patients for risk factors associated with IIE before initiating ifosfamide therapy. A comprehensive assessment will help identify those at higher risk for developing IIE.
Develop risk-based guidelines: We suggest the development of a risk-based guideline to enhance the management of IIE. Such a guideline would categorize patients based on their risk levels and provide tailored strategies for prevention and monitoring.
Preventive use of methylene blue: Consider administering methylene blue as a preventive measure in patients identified as high risk for IIE. This approach may help reduce the likelihood of developing encephalopathy.
Monitor for IIE symptoms: Pediatric patients undergoing ifosfamide therapy should be closely monitored for early signs of IIE. Prompt detection and management are crucial for effective intervention.
Therapeutic use of methylene blue: In the event that IIE occurs, Methylene Blue should be used as a therapeutic agent to alleviate and reverse symptoms. Our case supports the use of a dosage of 1 mg/kg (max 50 mg) administered intravenously, demonstrating effective symptom resolution within 12 hours. The dose can be repeated every 4 - 8 hours until neurotoxic symptoms are alleviated.