1. Background
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder and one of the most common psychiatric disorders in the child and adolescent population, with a prevalence rate of 4 - 9% (1). It has significant impacts on health services and the community, both economically and socially (2). Over the last decade, various guidelines have been developed for the treatment and management of this disorder. Stimulant medications are considered the most effective treatment for ADHD, with an acceptable efficacy and side effect profile. Clonidine, guanfacine, and atomoxetine are other approved drugs used for ADHD. However, approximately 30% of patients show resistance to these treatments (3).
When patients with ADHD do not respond to treatment with stimulants, other drug groups should be considered. Research has demonstrated that Alpha-2 agonists, such as clonidine and guanfacine, are effective in managing ADHD symptoms in children, and many studies have confirmed the efficacy and safety of these drugs in children and adolescents (4). In addition to their efficacy, Alpha-2 agonist drugs have advantages regarding side effects compared to stimulants and atomoxetine. Methylphenidate and other stimulants, such as amphetamines, can cause side effects including loss of appetite, weight loss, insomnia, and growth suppression. Atomoxetine, a non-stimulant drug, may cause side effects such as liver toxicity, nausea, and vomiting. Conversely, Alpha-2 agonists are well-tolerated and have relatively milder side effects (4-6).
Tizanidine, a centrally acting Alpha-2 adrenergic receptor agonist, is indicated for pain and muscle spasticity management (7). It differs significantly from clonidine and guanfacine in terms of hypotensive and cardiovascular side effects, as it has mild and transient blood pressure-lowering effects. Tizanidine has been approved by the Food and Drug Administration (FDA) for the treatment of stroke, traumatic brain injury, various degenerative brain disorders, multiple sclerosis, and spinal cord injuries. It has shown benefits in managing various types of chronic pain, including chronic neck pain, migraine headaches, and lumbosacral pain, in both adults and the pediatric population (8). However, to date, the safety and efficacy of tizanidine in the treatment of ADHD symptoms have not been studied.
2. Objectives
The purpose of this study is to evaluate the effects of adding tizanidine to stimulants in the treatment of ADHD.
3. Methods
According to previous studies and the difference between two means formula (α = 5%, d = 2.5, and power = 80%), 40 ADHD patients aged 6 - 18 years, who had experienced at least one month of unsuccessful treatment with lisdexamfetamine (Vyvanse), participated in the study. These patients were recruited from outpatient units or admission wards of child psychiatry departments at hospitals affiliated with Isfahan University of Medical Sciences.
3.1. Inclusion Criteria
Clinical diagnosis of ADHD was made according to the diagnostic and statistical manual of mental disorders, 5th edition (DSM-5). Inclusion criteria included at least 4 weeks of unsuccessful treatment with lisdexamfetamine (Vyvanse), age between 6 - 18 years, completion of a consent form signed by parents/guardians, assent by adolescents, no history of current or previous cardiovascular disease, no history of receiving any antihypertensive drugs such as alpha or beta blockers, and no history of allergic reactions to drugs.
Exclusion criteria included unwillingness of the patient or family to continue participating in the study, any new physical illness altering the course of patient care, any change in physical or mental condition requiring urgent action, and any drug side effects from stimulants or tizanidine that could not be managed or treated.
This double-blind, placebo-controlled trial was conducted from October 2023 to June 2024 over three phases (0, 4, and 8 weeks). The study protocol was designed according to the ethical principles of the Helsinki Declaration and was approved by the Ethics Committee of Isfahan University of Medical Sciences (IR.ARI.MUI.REC.1402.135). The protocol was also registered in the Iranian Registry of Clinical Trials (IRCT20230619058530N2). All contributors adhered to the Helsinki ethical principles.
Randomization was conducted using an online computer program through random blocks, and study treatments were randomly assigned to placebo or treatment groups. Participants in the treatment group (group 1) received tizanidine (Actover, Iran) and lisdexamfetamine (Vyvanse) (Tadbir Kalaye Jam, IRAN), while participants in the placebo group (group 2) received placebo capsules (Isfahan Faculty of Pharmacy) in the same ratio. Vyvanse was started in both groups at 10 mg/day orally, titrated up to a maximum dose of 70 mg/day if needed. In group 1, tizanidine was prescribed with a starting dose of 1 mg/day orally, titrated up every 4 days as tolerated, without side effects, to a target dose of 4 mg/day in 2 - 3 divided doses.
Patients were evaluated for ADHD symptom severity, blood pressure, heart rate, electrocardiogram (ECG) changes, and other side effects of tizanidine during the 8-week study period. The diagnosis of ADHD and exclusion of other psychiatric disorders were confirmed by a child and adolescent psychiatrist using DSM-5 criteria. A semi-structured interview was conducted to complete the diagnostic process using the Kiddie schedule for affective disorders and schizophrenia (K-SADS), which provides reliable and valid psychiatric diagnoses in the Iranian population (9, 10).
Symptom severity was assessed using the CONNERS' Parent Rating Scale-48 (CPRS-48), a validated tool with strong psychometric properties in the Iranian population (11, 12). This scale contains 48 items in 5 domains, based on parent-reported behaviors, and evaluates the child’s emotional and behavioral states, including psychosomatic feelings, impulsive/hyperactive behavior, learning problems, conduct problems, and anxiety. Another scale used to evaluate psychiatric symptoms was the Swanson, Nolan, and Pelham Rating Scale (SNAP-IV), which measures the core symptoms of ADHD across 18 items, addressing three dimensions: Attention deficit, hyperactivity, and impulsivity. This scale also has adequate psychometric properties in the Iranian population (13, 14).
Clinical evaluations and necessary care were conducted during all visits. The ECG, blood pressure, and heart rate were monitored at baseline, after 4 weeks, and at the end of 8 weeks. All patients were monitored for common drug side effects, including dry mouth, dizziness, vertigo, headache, abdominal pain, sleep disturbance, palpitations, tics, skin rashes, and nasal congestion throughout the study.
3.2. Statistical Analysis
The collected data, prepared during the study using questionnaires, was analyzed using the statistical package for the social sciences (SPSS Inc., Chicago, USA) version 21 and presented as n (%) and mean ± standard deviation (SD). A P-value of < 0.05 was considered statistically significant. The chi-square test and independent t-test were employed based on the normal distribution of measurements, as determined by the Kolmogorov-Smirnov test. A repeated measures test was used for quantitative outcome measures after adjusting for confounding variables such as age and gender.
4. Results
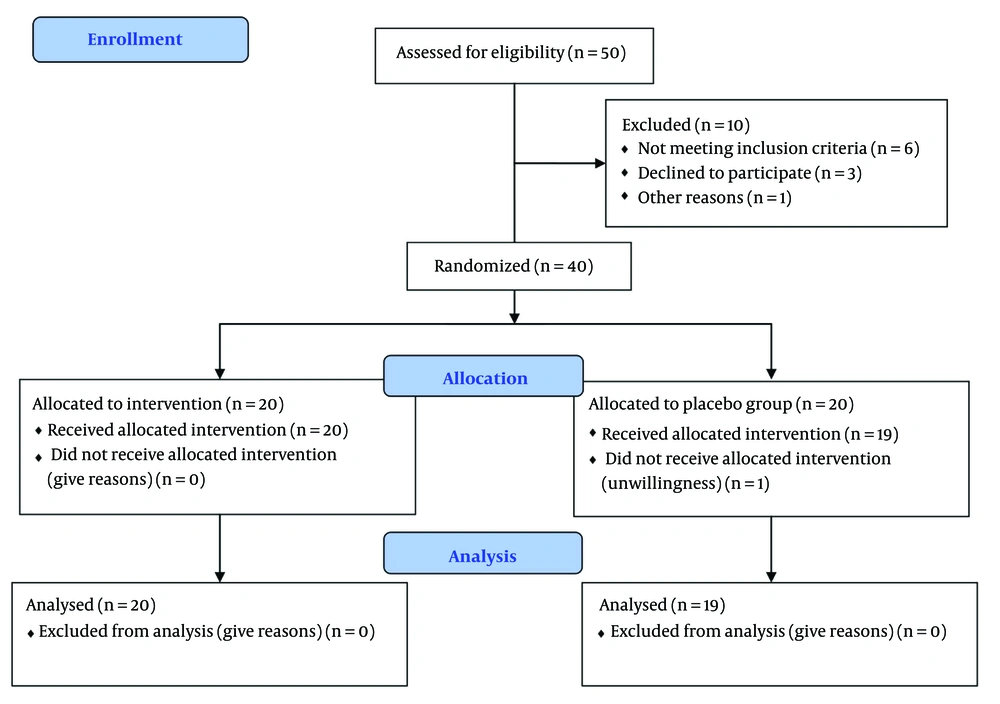
The participants included 40 patients with ADHD, aged between 6 - 18 years, who were equally divided into two groups: Tizanidine and placebo. One patient in the placebo group was excluded due to unwillingness to continue participation in the study (Figure 1). The average ages of participants who completed the trial in the tizanidine and placebo groups were 10.3 and 9.7 years, respectively. Among these, 70% and 78.9% were male in each group. According to statistical analysis, there was no significant difference in baseline characteristics such as age, gender, or educational status of the patients between the groups (Table 1).
After 8 weeks of study, there was no significant difference within and between groups in the severity of ADHD symptoms according to the SNAP-IV Scale (Table 2). Additionally, there was no statistically significant difference in the five domains of CPRS-48 between the tizanidine and placebo groups. However, a statistically significant difference was observed in the conduct domain within the tizanidine group from the start of the study to the end of week 8 (38 ± 8.7 vs. 43.5 ± 10.5, P < 0.05) (Table 3).
| Variables | Baseline | 4th Week | 8th Week | P-Value b | P-Value c |
|---|---|---|---|---|---|
| Inattention | 0.1 | ||||
| Tizanidine | 20.14 ± 0.8 | 1.9 ± 0.9 | 1.6 ± 0.9 | 0.9 | |
| Placebo | 1.6 ± 0.7 | 1.5 ± 0.8 | 1.4 ± 0.7 | 0.1 | |
| Hyperactivity | 0.9 | ||||
| Tizanidine | 1.75 ± 0.7 | 1.7 ± 0.6 | 1.5 ± 0.6 | 0.9 | |
| Placebo | 1.5 ± 0.6 | 1.4 ± 0.6 | 1.3 ± 0.6 | 0.1 | |
| Inattention/hyperactivity | 0.6 | ||||
| Tizanidine | 1.76 ± 0.6 | 1.7 ± 0.5 | 1.6 ± 0.5 | 0.9 | |
| Placebo | 1.5 ± 0.6 | 1.4 ± 0.6 | 1.4 ± 0.6 | 0.3 |
Changes of Swanson, Nolan, and Pelham Rating Scales During the Study and the Result of Longitudinal Analysis a
| Variables | Baseline | 4th Week | 8th Week | P-Value b | P-Value c |
|---|---|---|---|---|---|
| Conduct domain | 0.07 | ||||
| Tizanidine | 43.5 ± 10.5 | 38.75 ± 9.2 | 38 ± 8.7 | 0.03 d | |
| Placebo | 39.4 ± 8 | 39 ± 6.6 | 37.4 ± 7 | 0.5 | |
| Social domain | 0.5 | ||||
| Tizanidine | 24.6 ± 5.5 | 23.3 ± 5.6 | 22.7 ± 4.9 | 0.3 | |
| Placebo | 22.1 ± 4.8 | 20.5 ± 4.3 | 20.3 ± 4 | 0.26 | |
| Psychosomatic domain | 0.5 | ||||
| Tizanidine | 21.6 ± 6 | 20.3 ± 5.8 | 19.9 ± 5.4 | 0.1 | |
| Placebo | 18.6 ± 5.2 | 18.3 ± 4.9 | 17.9 ± 4.9 | 0.07 | |
| Anxiety domain | 0.5 | ||||
| Tizanidine | 15.1 ± 4.3 | 14 ± 4 | 13.9 ± 3.9 | 0.73 | |
| Placebo | 14.7 ± 3.8 | 14.7 ± 4.1 | 13.6 ± 3.7 | 0.1 | |
| Total | 0.2 | ||||
| Tizanidine | 108.7 ± 22.7 | 100.7 ± 20.9 | 99 ± 19.5 | 0.2 | |
| Placebo | 96.4 ± 16.4 | 93.9 ± 14.6 | 91.4 ± 3.8 | 0.4 |
Changes of CONNERS' Parent Rating Scale-48 Questionnaire Scales During the Study and the Result of Longitudinal Studies a
4.1. Safety Measurement
Safety evaluation of participants was conducted regularly for all individuals who received the drug at least once after randomization. Mean changes in blood pressure, pulse rate, and ECG indexes such as PR interval, QTc (corrected) segment, and QRS duration from baseline to week 8 were compared between the two groups (Table 4). None of the participants showed abnormal ECG findings at baseline, week 4, or week 8 in either treatment group.
| Variables | Baseline | 4th Week | 8th Week | P-Value b |
|---|---|---|---|---|
| Pulse rate | 0.3 | |||
| Tizanidine | 86.9 ± 14.3 | 89.6 ± 15 | 92.4 ± 3.6 | |
| Placebo | 91.9 ± 17.7 | 91.2 ± 14.1 | 91.5 ± 10.2 | |
| BPsystolic | 0.4 | |||
| Tizanidine | 96.5 ± 9.3 | 94.3 ± 9.9 | 97.5 ± 7.7 | |
| Placebo | 94.2 ± 10.7 | 92.6 ± 10.6 | 91.6 ± 11.8 | |
| BPdiastolic | 0.9 | |||
| Tizanidine | 62 ± 7.7 | 61.3 ± 6.4 | 62 ± 5.2 | |
| Placebo | 60.5 ± 7.8 | 60 ± 10.1 | 60.8 ± 8 | |
| QTc (corrected) milliseconds | 0.5 | |||
| Tizanidine | 379.9 ± 34.5 | 379.2 ± 27.8 | 378.5 ± 19.7 | |
| Placebo | 381.8 ± 22.7 | 371 ± 16 | 375.8 ± 21.5 | |
| PR interval (milliseconds) | 0.3 | |||
| Tizanidine | 108.6 ± 29 | 113.7 ± 25.8 | 113.7 ± 26.6 | |
| Placebo | 106.4 ± 36.7 | 108.5 ± 33 | 103.2 ± 29.5 | |
| QRS (milliseconds) | 0.3 | |||
| Tizanidine | 77 ± 24.1 | 77.1 ± 23.3 | 74 ± 25.7 | |
| Placebo | 48.4 ± 14.6 | 44.2 ± 14.3 | 46.3 ± 14.2 |
Changes of Blood Pressure and Electrocardiogram Scales During the Study and the Result of Longitudinal Analysis a
In the tizanidine and placebo groups, the QTc value did not show a statistically significant change at weeks 4 and 8 compared to the beginning of the study (378.5 ± 19.7 vs. 375.8 ± 21.5). There was no significant difference within or between the two groups at baseline, week 4, and week 8 measurements for blood pressure and pulse rate (Table 4).
5. Discussion
Attention deficit hyperactivity disorder is a common psychiatric disorder in childhood and adolescence. The core symptoms of ADHD include hyperactivity, impulsivity, and difficulty with attention and concentration. These symptoms can significantly impact the academic and social aspects of a child's life. The efficacy of Alpha-2 agonists in treating hyperactivity, impulsivity, and inattention in children with ADHD has been demonstrated in various studies. For example, a randomized double-blinded placebo-controlled clinical trial in 2013 showed that guanfacine is effective in controlling ADHD symptoms in the pediatric population. Another study in 2018 found that clonidine can be effective in improving ADHD and tic symptoms in children aged 6 - 12 years (6). Therefore, this class of drugs can be a good option for children who do not respond well to stimulants, experience side effects from stimulants, or have tic disorders co-occurring with ADHD. In general, these drugs are well tolerated by patients and have an acceptable side effect profile.
This study with tizanidine was conducted for the first time on ADHD patients, highlighting the need for more detailed research on this population, as no other study has assessed the effectiveness of this drug in ADHD or other psychiatric disorders. In this study, after 8 weeks of treatment, there was no statistically significant difference in the severity of ADHD symptoms between the study groups. This lack of significant results may be due to the low dose of tizanidine used or the baseline resistance of patients to the treatment regimen. Additionally, the relatively small sample size may have limited the ability to draw decisive conclusions.
However, tizanidine was found to be effective in reducing conduct behaviors. This finding is consistent with existing evidence that Alpha-2 agonist drugs, such as clonidine and guanfacine, are effective in reducing aggression and oppositional behaviors in ADHD patients with comorbid conduct disorders (15). Similar studies have shown that aggression is reduced by Alpha-2 agonists (16), suggesting that this class of drugs may serve as an alternative treatment for comorbid ADHD and conduct disorder. This is especially relevant when considering the advantage of Alpha-2 agonists over antipsychotics, as they lack antipsychotic-related side effects such as extrapyramidal and metabolic complications. Evidence also suggests that Alpha-2 agonists have a modest effect on oppositional behavior but are effective in reducing aggression in conduct disorder (15). Additionally, these drugs are approved for managing behavioral disturbances in autism spectrum disorder (17).
This study also demonstrated that tizanidine has a favorable safety profile in terms of cardiovascular complications in children and adolescents, as the incidence of side effects in the tizanidine group was comparable to the placebo group. This finding is consistent with previous studies (18, 19). Therefore, tizanidine may be considered a safe medication for use in children and adolescents.
5.1. Conclusions
According to this study, while tizanidine demonstrates good safety in the pediatric age group, it is not effective for the treatment of ADHD symptoms in this population. Further studies are needed to evaluate the efficacy of this drug in managing behavioral disorders.
5.2. Limitations
Whereas this study showed that tizanidine is not effective in controlling ADHD symptoms, it should be noted that to confirm this finding, studies with larger sample sizes are necessary, as the sample size in this study was limited. Additionally, the study period should be extended in future research to provide more comprehensive insights.