1. Background
Even with advancements in neonatal intensive care, pulmonary hemorrhage (PH) remains a serious and often fatal complication in very low birth weight (VLBW) infants, continuing to be a major cause of morbidity and mortality in this vulnerable population (1).
Patent ductus arteriosus (PDA) is a significant risk factor for PH in VLBW infants and can influence its occurrence through several mechanisms. First, the persistence of PDA may lead to a large left-to-right shunt, resulting in increased pulmonary blood flow. The fragile and immature pulmonary vasculature in VLBW infants may become overwhelmed by this heightened blood flow, leading to vascular rupture and subsequent hemorrhage (2).
Additionally, PDA can worsen existing lung conditions, such as respiratory distress syndrome, which is another risk factor for PH. The increased pulmonary blood flow caused by PDA can lead to pulmonary edema, further impairing lung function. This can escalate the need for mechanical ventilation and oxygen therapy, both of which are known contributors to the development of PH (3).
Therefore, early identification and management of PDA in VLBW infants are essential to reducing the risk of PH and improving neonatal outcomes. However, the optimal strategy for managing PDA—whether through expectant management with supportive measures or early pharmacologic therapy using cyclooxygenase (COX) inhibitors—remains a subject of ongoing research and debate (4).
We hypothesized that the pharmacological treatment of hemodynamically significant PDA (hs-PDA) would increase ductal closure rates and reduce the incidence of PH compared to supportive care alone in VLBWinfants. The primary research questions were as follows:
(1) Does pharmacological treatment improve PDA closure rates in hs-PDA?
(2) Is there a relationship between PDA closure and PH incidence?
(3) Does hs-PDA correlate with an increased risk of PH?
At our center, early pharmacologic therapy is initiated within the first three days after birth for VLBW neonates with hs-PDA, based on the two most common echocardiographic criteria: A PDA diameter > 1.5 mm and/or left atrial enlargement (5).
2. Objectives
The aim of this study was to evaluate the prevalence of PDA closure following the administration of pharmacologic therapy for hs-PDA, as determined by the specified echocardiographic criteria, and to assess its effect on the frequency of PH in VLBW infants.
3. Methods
3.1. Design
This retrospective cohort study was conducted between March 2018 and March 2022 at Firoozabadi Teaching Hospital in Tehran, Iran, following approval from the Research Ethics Committee of Iran University of Medical Sciences (IR.IUMS.REC.1401.852).
3.2. Participants
3.2.1. Inclusion Criteria
- Birth weight < 1500 g
- The PDA identified on first echocardiography (within three days after birth)
- Admission within first 24 hours of life
- Parental consent for data use in research
3.2.2. Exclusion Criteria
- Major congenital anomalies
- Incomplete medical records
- Transfer to other facilities within the study period
3.3. Study Protocol
Information about the infants was obtained from their medical records with parental consent and entered into a standardized data collection form. Baseline demographic data included gestational age, birth weight, gender, Apgar scores, and the presence of intrauterine growth restriction.
The infants were categorized into two groups based on echocardiographic findings:
3.3.1. Non-hs-PDA Group
Treated with general supportive measures only.
3.3.2. hs-PDA Group
Received pharmacological treatment according to the protocol.
Hemodynamically significant PDA (hs-PDA) was defined using the following echocardiographic criteria:
- The PDA diameter > 1.5 mm and/or
- Left atrial enlargement (LA:Ao ratio > 1.4).
3.4. Treatment Protocol
For the hs-PDA group, intravenous acetaminophen was administered according to the following protocol:
- Dose: 15 mg/kg/dose
- Frequency: Every 6 hours
- Duration: Three consecutive days
- Initiation: Within 72 hours of birth
Follow-up echocardiography was performed three days after the initial examination in both groups to evaluate PDA status. All infants were closely monitored for the occurrence of PH during the first week of life.
3.5. Outcome Measures
3.5.1. Primary Outcomes
- The PDA closure rate following treatment
- Incidence of PH
3.5.2. Secondary Outcomes
- Duration of mechanical ventilation
- Development of bronchopulmonary dysplasia (BPD)
- Intraventricular hemorrhage (IVH)
- Necrotizing enterocolitis (NEC)
- Mortality before discharge
3.6. Statistical Analysis
The sample size was calculated based on previously reported PDA closure rates, with α = 0.05 and β = 0.20, requiring a minimum of 40 participants to detect a 30% difference in closure rates between groups.
Data analysis was conducted using SPSS version 26.0. Continuous variables were tested for normality using the Shapiro-Wilk test. Normally distributed variables were compared using independent t-tests, while non-normally distributed data were analyzed with the Mann-Whitney U test. Categorical variables were compared using Fisher's exact test or chi-square test, as appropriate.
Multivariate logistic regression analysis was performed to identify independent predictors of PH, adjusting for potential confounders such as gestational age, birth weight, and mechanical ventilation duration. A P-value of < 0.05 was considered statistically significant for all analyses.
The relationship between PDA closure and PH was examined using time-to-event analysis. Risk ratios with 95% confidence intervals were calculated for the primary outcomes.
4. Results
4.1. Study Population
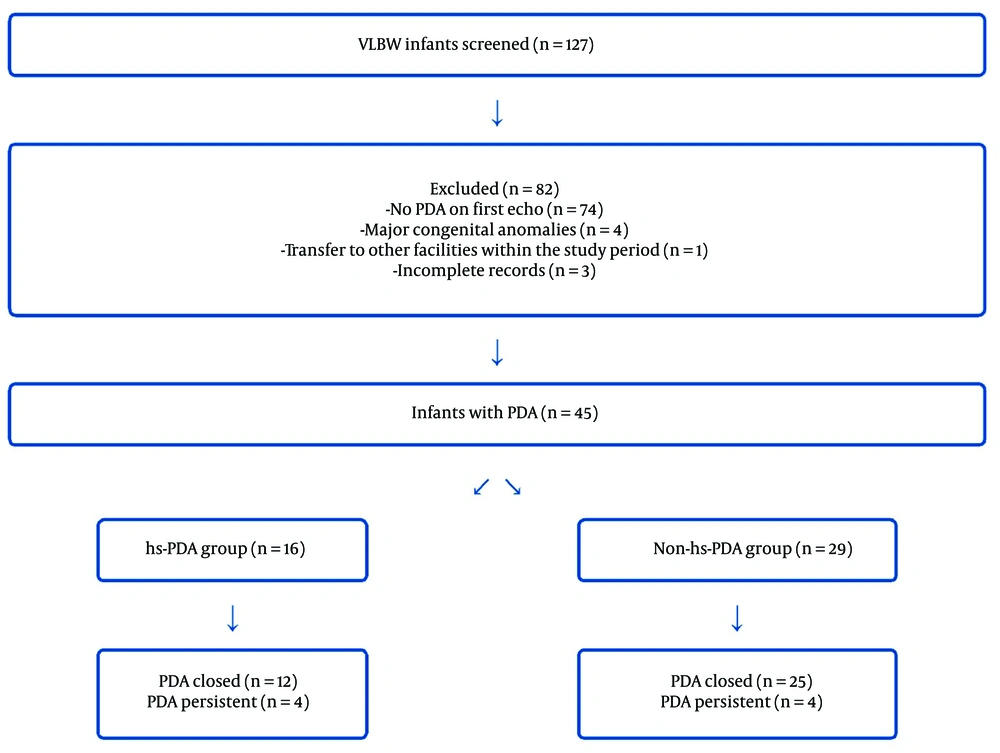
Of the 127 VLBW infants screened during the study period, 45 were found to have PDA on their initial echocardiography. After applying the inclusion and exclusion criteria, these infants were categorized into two groups: hs-PDA (n = 16) and non-hs-PDA (n = 29). Figure 1 illustrates the flow of participants through the study.
4.2. Baseline Characteristics
As presented in Table 1, baseline characteristics were comparable between the groups. The mean gestational age was 28.69 ± 1.81 weeks in the hs-PDA group and 29.48 ± 3.19 weeks in the non-hs-PDA group (P = 0.36). Mean birth weights were also similar (1186 ± 251 g vs. 1187 ± 235 g, P = 0.99). No significant differences were noted in gender distribution (56.3% vs. 48.3% male, P = 0.60), antenatal steroid administration (81.3% vs. 82.8%, P = 0.89), cesarean delivery rates (75.0% vs. 75.9%, P = 0.95), or surfactant therapy (87.5% vs. 79.3%, P = 0.48).
| Characteristic | hs-PDA Group (n = 16) | Non-hs-PDA Group (n = 29) | P-Value |
|---|---|---|---|
| Gestational age (wk) | 28.69 ± 1.81 | 29.48 ± 3.19 | 0.36 |
| Birth weight (g) | 1186 ± 251 | 1187 ± 235 | 0.99 |
| Male gender | 9 (56.3) | 14 (48.3) | 0.60 |
| IUGR | 0 (0) | 2 (6.9) | 0.28 |
| Antenatal steroids | 13 (81.3) | 24 (82.8) | 0.89 |
| Cesarean delivery | 12 (75.0) | 22 (75.9) | 0.95 |
| 5-min Apgar score | 7.2 ± 1.4 | 7.4 ± 1.3 | 0.63 |
| Surfactant therapy | 14 (87.5) | 23 (79.3) | 0.48 |
a Values are expressed as mean ± SD or No. (%).
4.3. Patent Ductus Arteriosus Treatment Outcomes
In the hs-PDA group, all infants received intravenous acetaminophen as per protocol, with PDA closure achieved in 7 patients (43.8%) following treatment. In the non-hs-PDA group, spontaneous closure occurred in 15 patients (51.7%). The difference in closure rates between the groups was not statistically significant (P = 0.43).
4.4. Pulmonary Hemorrhage and Clinical Outcomes
As shown in Table 2, PH occurred more frequently in the hs-PDA group compared to the non-hs-PDA group (37.5% vs. 17.2%, P = 0.04). Residual PDA was significantly associated with PH, whereas the initial presence of hs-PDA alone did not predict PH development.
| Outcome | hs-PDA Group (n = 16) | Non-hs-PDA Group (n = 29) | P-Value |
|---|---|---|---|
| PDA closure | 7 (43.8) | 15 (51.7) | 0.43 |
| Pulmonary hemorrhage | 6 (37.5) | 5 (17.2) | 0.04 |
| Mechanical ventilation (days) | 12 ± 75 | 18 ± 62.1 | 0.378 |
| BPD | 5 (31.3) | 7 (24.1) | 0.600 |
| IVH | 10 (62.5) | 15 (51.7) | 0.486 |
| NEC | 5 (31.3) | 0 (0) | 0.001 |
| Mortality | 4 (25) | 8 (27.6) | 0.851 |
Abbreviations: PDA, patent ductus arteriosus; BPD, bronchopulmonary dysplasia; IVH, Intraventricular hemorrhage; NEC, Necrotizing enterocolitis.
a Values are expressed as mean ± SD or No. (%).
Secondary outcomes revealed no significant differences between the groups in rates of mechanical ventilation (75% vs. 62.1%, P = 0.378), bronchopulmonary dysplasia (BPD) (31.3% vs. 24.1%, P = 0.600), or IVH (62.5% vs. 51.7%, P = 0.486). However, NEC was significantly more common in the hs-PDA group (31.3% vs. 0%, P = 0.001). Mortality rates were similar between the groups (25% vs. 27.6%, P = 0.851).
5. Discussion
Our study's investigation into the relationship between PDA treatment and PH in VLBW infants has revealed several significant findings that merit detailed discussion. These results contribute to the ongoing debate regarding optimal PDA management strategies in preterm infants and raise important questions about the adequacy of current treatment protocols.
5.1. Treatment Outcomes and Clinical Implications
The observation that pharmacological treatment of hs-PDA did not significantly improve ductal closure rates compared to supportive care alone (43.8% vs. 51.7%, P = 0.43) aligns with recent paradigm shifts in PDA management. This finding supports accumulating evidence from larger studies, such as the PDA-TOLERATE trial by Clyman et al., which questioned the benefit of routine pharmacological closure of PDA (6). Similarly, the BeNeDuctus trial demonstrated that early treatment strategies may not offer superior outcomes compared to expectant management (7).
The comparable closure rates between treated and untreated groups in our study raise critical questions about current treatment protocols. A study by El-Khuffash et al. reported similar findings, where early routine treatment failed to improve clinical outcomes, suggesting the need for more refined treatment criteria (8). This pattern reinforces the growing perspective in neonatal care that not all PDAs require active intervention, and a more selective approach to treatment may be more appropriate.
5.2. Hemodynamic Significance and Clinical Outcomes
Our finding that residual PDA was significantly associated with PH, whereas initial hs-PDA status alone was not predictive, provides crucial insights into PDA management. This observation aligns with recent studies suggesting that echocardiographic criteria for assessing PDA significance may require substantial revision (9, 10). The distinction between initial PDA size and persistence is particularly relevant for clinical decision-making, as it implies that the duration of exposure to ductal shunting may be more critical than the initial measurements.
The higher incidence of NEC in the hs-PDA group (31.3% vs. 0%, P = 0.001) is a concerning finding that warrants particular attention. This association, previously documented by Haefeli et al., may be attributed to altered mesenteric blood flow patterns in the presence of significant PDA (11). Further supporting this relationship, Smith et al.'s work suggests that the impact of PDA on systemic perfusion may have more far-reaching consequences than previously understood (12).
5.3. Mortality and Long-term Outcomes
The similar mortality rates observed between the groups (25% vs. 27.6%, P = 0.851) are consistent with recent meta-analyses by Mitra et al., which questioned the impact of early PDA treatment on survival outcomes in VLBW infants (13). This finding supports the growing trend toward more conservative management approaches in non-symptomatic infants, as demonstrated by Okulu et al. (14). The challenge remains in identifying which infants would genuinely benefit from intervention, necessitating a careful balance between the risks associated with treatment and the potential complications of persistent PDA.
5.4. Impact of Contemporary Healthcare Challenges
The study period's overlap with the COVID-19 pandemic introduces unique considerations for interpreting our results. As documented by Salvatore et al., the pandemic has significantly impacted various aspects of neonatal care delivery (15). This context is crucial when evaluating the generalizability of our findings and their potential application to future clinical practice.
5.5. Future Directions in Patent Ductus Arteriosus Management
The evolution of PDA management strategies, as outlined by van Laere et al., highlights the need for more comprehensive assessment tools that extend beyond echocardiographic parameters (16). Joye et al.'s research on targeted neonatal echocardiography services underscores the importance of standardized assessment approaches (17). Together with our findings, these developments point to several key areas for future research and practice improvement:
5.5.1. Refined Assessment Criteria
- Development of multimodal assessment protocols incorporating clinical, biochemical, and echocardiographic parameters
- Integration of advanced imaging techniques for better prediction of PDA-related complications
- Validation of new biomarkers for PDA significance
5.5.2. Treatment Optimization
- Investigation of personalized treatment approaches based on individual risk profiles
- Evaluation of alternative therapeutic strategies
- Development of predictive models for treatment response
5.5.3. Long-term Outcomes
- Studies on the long-term impact of different management strategies
- Assessment of neurodevelopmental outcomes in relation to PDA management decisions
- Investigation of potential late complications of both treated and untreated PDA
Gowda et al.'s work on refined approaches to ductal management provides a valuable framework for these future directions (18). Chan and Singh have further emphasized the necessity of developing evidence-based diagnostic and treatment protocols (19), while Mitra et al.'s research underscores the critical role of early screening and risk stratification in improving outcomes (20).
5.6. Study Limitations and Methodological Considerations
Several limitations should be considered when interpreting our results:
5.6.1. Study Design
- The retrospective nature limits causal inference
- Potential selection bias in treatment allocation
- Limited ability to control for confounding variables
5.6.2. Sample Size and Power
- Relatively small sample size, particularly in the hs-PDA group
- Possible type II errors in secondary outcome analyses
- Limited subgroup analysis capabilities
5.6.3. External Validity
- Single-center study design affecting generalizability
- Local treatment protocols may differ from other institutions
- Population-specific factors may influence outcomes
5.6.4. Temporal Considerations
- Evolution of treatment protocols during the study period
- Impact of the COVID-19 pandemic on care delivery
- Changes in clinical practice patterns over time
5.7. Conclusions
This study demonstrates that while pharmacological treatment of hs-PDA based solely on echocardiographic criteria did not significantly improve ductal closure rates, persistent PDA was associated with an increased risk of pulmonary hemorrhage. These findings suggest that current treatment strategies may require refinement, emphasizing the need to identify infants who are most likely to benefit from intervention. Future research should focus on developing more comprehensive criteria to guide treatment decisions and exploring alternative management strategies for PDA in VLBW infants.