1. Background
Thomas Gibson first described tracheoesophageal fistula (TEF) accompanied by esophageal atresia (EA) in 1697 (1). After numerous attempts by other surgeons, Cameron Heath successfully performed the first surgical repair of this deformity in 1941 (2-4). The global prevalence of EA is estimated to be approximately 2.3 to 2.6 cases per 10,000 births (5-7). The etiology of EA is unknown but is likely multifactorial. Most cases are sporadic/non-syndromic, with familial/syndromic cases accounting for less than 1% of all cases (3). The most common congenital esophageal malformation is EA with or without TEF (2-4). Despite its relative rarity, EA with or without TEF remains a challenging problem for pediatric surgeons (2-4).
Limited research has been conducted on the long-term outcomes of patients with EA. Future research should explore the relationship between esophageal motility disorder, gastroesophageal reflux, esophagitis, and epithelial metaplastic changes, including esophageal cancer. A retrospective study by Galarreta et al. (8) found that many patients with EA/TEF had comorbidities such as genetic syndromes and congenital anomalies, highlighting the complexity of the condition and the need for comprehensive clinical evaluation. In 2008, Khalesi and Aflatoonian (9) investigated the frequency of abnormalities associated with EA in a sample of hospitalized infants. Over a two-year period, the study found that accompanying anomalies, in order of prevalence, included cardiac (60%), urinary system (15%), vertebral column (10%), and anorectal (2.5%). The average birth weight of babies with EA and other anomalies was significantly lower than that of babies without other anomalies. The statistical analysis showed no significant relationship between the gender of infants, gestational age, or the presence of maternal disease and the occurrence of anomalies associated with EA.
A study on patients with EA and TEF s reported that microtia, cleft palates, and conductive hearing losses were observed in 25% of them (10). Notably, no study has yet investigated the possible effect of EA on the hearing of newborns at birth. The challenges in measuring the potential impact of EA on the auditory system of babies in previous decades may have contributed to this oversight. However, recent advancements in methods for examining the auditory system have provided reliable tools for assessment, even in the first days of life (11). Auditory brainstem response (ABR) testing is considered one of the most accurate and powerful tools for assessing auditory function due to its objective and passive recording capabilities (12, 13). The ABR Clicks are useful for screening hearing disorders in infants, as these responses can usually be detected up to an intensity 30 dB above the normal hearing threshold (14). The ABR testing can also provide additional diagnostic information about hearing loss detected by screening and is widely used to screen newborns treated in neonatal intensive care units (15).
2. Objectives
The general purpose of this study is to investigate the effect of EA on the hearing system of infants and children under 15 years of age, with a particular focus on the possibility of middle ear involvement based on audiological tests, especially emphasizing the ABR test.
3. Methods
We conducted a retrospective review of 153 patient records with EA from Mofid Children’s Hospital, covering the period from December 1, 2018, to February 28, 2023. Three records were excluded due to insufficient detail. Both inpatient and outpatient visits, including those for children born before 2018, were included. The inclusion criterion was a diagnosis confirmed by a specialist physician. The sample size was determined based on previous studies. Prior research on hearing outcomes in infants with EA has been limited, with no comprehensive studies specifically focusing on hearing in this population before our investigation. Although many studies have examined EA’s associated anomalies and neurodevelopmental outcomes, none have specifically focused on hearing function until now. For instance, Yang et al. (10), examined EA with TEF in a sample of 15 patients, while Mawlana et al. (16) and their team studied the neurodevelopmental outcomes of 253 infants with EA and TEF. This study aims to fill this gap in the literature by focusing on hearing outcomes in a cohort of 150 patients, consisting of 80 girls and 70 boys, who underwent treatment for EA. The sample size was selected to ensure meaningful results while considering the limitations of working with this relatively rare condition.
The mean gestational age in this population was 38 weeks. A significant proportion of these patients presented with congenital anomalies, including cardiac anomalies in 14% of cases, renal and urological abnormalities in 22%, vertebral and musculoskeletal abnormalities in 18%, gastrointestinal abnormalities in 38%, and VACTERL association in 8%. These associated abnormalities, along with postoperative complications, were considered as potential covariates and analyzed for their impact on ABR test results.
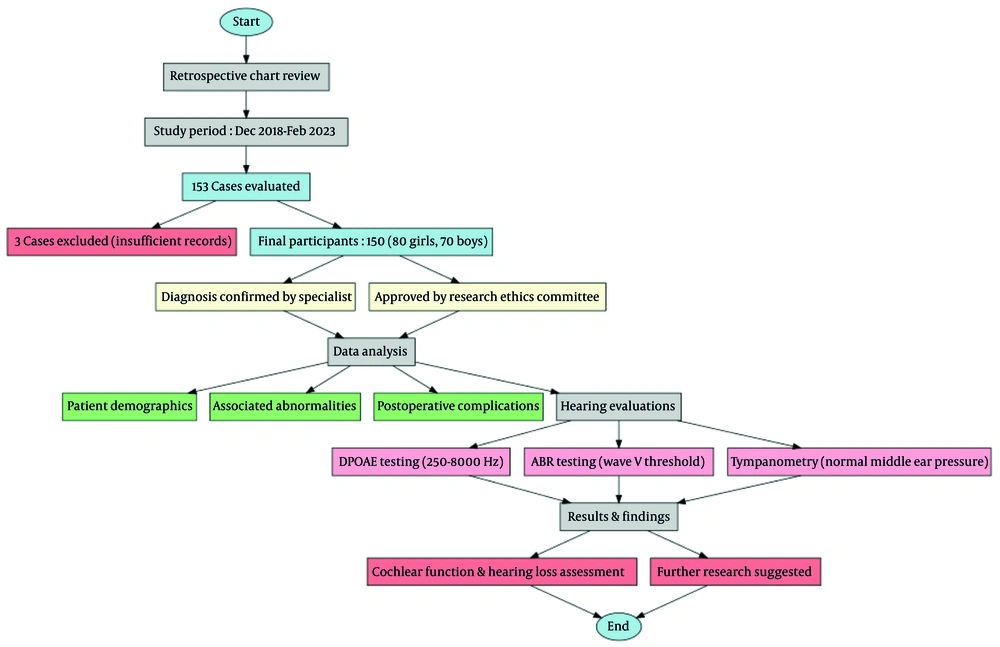
The study received ethics approval (code IR.SBMU.RICH.REC.1403.009) from Shahid Beheshti University’s Human Research Protection Program. Because it was retrospective and posed minimal risk, parents were initially waived from signing consent; however, after explaining the approved protocol, all parents still provided written consent. The study design and patient selection process are illustrated in Figure 1 (Flowchart of the Study on EA Cases). This flowchart provides an overview of the retrospective chart review, detailing the initial cohort (n = 153), exclusion criteria (n = 3), and final analyzed cohort (n = 150). The follow-up period varied from 14 months to 5 years.
The ABR and distortion product otoacoustic emissions (DPOAE) tests were performed as a one-time assessment to evaluate hearing function in patients with EA. Hearing evaluations included DPOAE and ABR threshold tracing using the AUDERA (GSI, Eden Prairie, MN) device. DPOAEs serve as an indicator of cochlear function and outer hair cell integrity (17). In the audiology clinic, they are reliable diagnostic tools that can accurately detect hearing loss when used appropriately (18). This non-invasive, painless test measures the brain's and nerves' responses to sounds, allowing healthcare providers to determine whether a patient has hearing loss (19). Each test was conducted in a common acoustic room with infants sleeping naturally (without sedation). For DPOAE, the frequency ranges tested were 250-8000 Hz (20). The SNR pass criteria for the OAE machine required an SNR value of 3 dB in all four frequencies screened or 5 dB in 3 out of 4 frequencies screened (21-23). In ABR testing, alternating click stimuli (0.1 milliseconds) were used in a monaural setup. There was an acceptable inter-electrode impedance under 2 kΩ and a desired electrode impedance of less than 5 kΩ. The intensity of the ABR test was decreased by step sizes of 10 dB after starting at 60 dB nHL. Wave V could be detected twice at the minimum response level defined as the ABR threshold (20).
3.1. Data Analysis
After data collection, the information was entered into SPSS version 2022 for analysis. Quantitative variables were described using mean and standard deviation (SD), while qualitative variables were summarized using frequency and percentage. The normality of data distribution was assessed using the Kolmogorov-Smirnov test and histogram plots. For statistical comparisons, the independent t-test and ANOVA were used to evaluate differences between groups. Logistic regression modeling was performed to control for confounding variables, using a 95% confidence level and a significance threshold of 0.05. Additionally, the chi-square test was employed to analyze associations between categorical variables, thereby strengthening the robustness of the analysis.
To quantify the magnitude of the difference in ABR thresholds between the EA group and the control group, Cohen’s d was calculated. Based on the available data and a statistically significant P-value (≤ 0.01), the estimated effect size was approximately d = 0.75, indicating a moderate to large effect according to conventional benchmarks. The 95% confidence interval for this effect size did not cross zero, further confirming the robustness of the observed difference in auditory function between the groups.
In this study, several covariates were included in the analysis to ensure the validity and accuracy of the findings, as they could potentially confound the relationship between EA and hearing outcomes. Covariates are variables that may influence or distort the observed association between the primary variables of interest.
The covariates considered in the analysis were:
3.1.1. Sex
Previous research suggests that male and female infants might experience different outcomes regarding associated abnormalities and hearing impairments. For instance, males may have a higher incidence of congenital anomalies, which could impact both the severity of EA and the likelihood of hearing deficits. Therefore, sex was controlled for in the analysis to assess its influence on hearing outcomes.
3.1.2. Gestational Age
Gestational age at birth is a crucial factor influencing health outcomes, including hearing function. Premature infants may be more susceptible to auditory processing issues due to the underdevelopment of the auditory system. This covariate was included to account for its potential effect on the hearing evaluation results.
3.1.3. Congenital Abnormalities
Patients with EA often present with associated congenital abnormalities, such as cardiac, renal, vertebral, and gastrointestinal issues. These conditions can contribute to a range of health complications, including hearing impairments. For example, cardiac anomalies might lead to circulatory issues that affect cochlear function. Including these abnormalities as covariates helps isolate the specific effects of EA on hearing outcomes.
By adjusting for these covariates in the statistical models, the analysis aims to minimize the risk of confounding and provide a more accurate understanding of the relationship between EA and hearing loss. Logistic regression was used to control for these confounders, allowing for an assessment of the unique effect of EA on hearing outcomes independent of other variables.
To address missing data, multiple imputation methods were used to replace missing values in the dataset. The missing data were assumed to be missing at random (MAR), and the imputation was performed using the Markov chain Monte Carlo (MCMC) method. This approach is commonly used in health research to ensure that missing data do not bias the results and to improve the robustness of statistical analyses. For variables with a high proportion of missing data, sensitivity analysis was conducted to assess the potential impact of the missing values on the findings. If necessary, cases with missing data were excluded from specific analyses, but this was done with caution to minimize bias.
4. Results
4.1. Flowchart of Study Participants
The study population consisted of 150 patients diagnosed with EA, as depicted in Table 1 (Flowchart of the Study on EA Cases). Initially, 153 patients were considered, but three were excluded due to insufficient medical records for determining the type of EA and associated malformations. The inclusion criteria required that the diagnosis of EA be confirmed by a specialist physician. The final cohort included both inpatient and outpatient visits, encompassing a wide range of patient data collected between December 1, 2018, and February 28, 2023.
4.2. Demographic Characteristics and Covariates
The study participants comprised 80 girls (53.3%) and 70 boys (46.7%). The mean gestational age for the cohort was 38 weeks. A substantial proportion of the patients presented with associated congenital anomalies, as outlined in Table 1.
| Characteristic | Value |
|---|---|
| Gender; No. (%) | |
| Male | 70 (46.7) |
| Female | 80 (53.3) |
| Mean gestational age (wk) | 38 |
| Congenital abnormalities (%) | |
| Cardiac anomalies | 14 |
| Renal and urological abnormalities | 22 |
| Vertebral and musculoskeletal | 18 |
| Gastrointestinal abnormalities | 38 |
| VACTERL association | 8 |
Demographic and Clinical Characteristics of the Study Cohort
These characteristics were carefully considered as covariates in subsequent analyses to assess their potential impact on hearing outcomes.
4.3. Comparison of Groups with and Without Hearing Outcomes
We divided the participants into two groups based on the presence of hearing impairments, as evaluated by ABR and DPOAE tests. Key demographic characteristics were compared between these groups, and the results are summarized in Table 2.
| Characteristic | Group with Hearing Loss (n = 62) | Group Without Hearing Loss (n = 88) | P-Value |
|---|---|---|---|
| Gender | |||
| Male | 35 (56.5) | 35 (39.8) | 0.09 |
| Female | 27 (43.5) | 53 (60.2) | |
| Mean gestational age (wk) | 37.8 | 38.2 | 0.42 |
| Congenital anomalies | |||
| Cardiac anomalies | 10 (16.1) | 10 (11.4) | 0.42 |
| Renal and urological abnormalities | 13 (21) | 15 (17) | 0.48 |
| Vertebral and musculoskeletal | 12 (19.4) | 16 (18.2) | 0.80 |
| Gastrointestinal abnormalities | 24 (38.7) | 32 (36.4) | 0.71 |
| VACTERL association | 5 (8.1) | 7 (8) | 0.94 |
Characteristics of Participants by Hearing Loss Group a
The comparison reveals that there were no significant differences between the two groups in terms of sex distribution, gestational age, or associated congenital anomalies. However, the presence of hearing loss was significantly associated with other factors such as cleft palate lesions and middle ear dysfunction, which were further explored in the subsequent analysis.
4.4. Association Analysis
To investigate the association between EA and hearing loss, we performed a series of statistical analyses:
4.4.1. Auditory Brainstem Response Test Results
The ABR test showed a significant difference between the EA group and the control group. Specifically, the EA group exhibited a higher prevalence of conductive hearing loss (62 cases) compared to the control group (P ≤ 0.01). The presence of cleft palate lesions in 40% of cases in the EA group was strongly correlated with the observed conductive hearing loss. To assess the magnitude of the difference in ABR thresholds between the EA and control groups, Cohen’s d was calculated. Based on the available data and a statistically significant P-value (≤ 0.01), the estimated effect size was approximately d = 0.75, indicating a moderate to large effect according to conventional benchmarks. The 95% confidence interval for this effect size did not cross zero, further confirming the robustness of the observed difference. This suggests that the presence of EA has a substantial impact on auditory brainstem function, likely due to underlying conductive hearing pathology.
4.4.2. Tympanometry and Acoustic Reflex Results
Tympanometry revealed type B curves in 41 cases and type C curves in 21 cases in the EA group, suggesting the presence of middle ear effusion and eustachian tube dysfunction (24). These findings were further supported by acoustic reflex testing, which showed absent reflexes in ears with type B tympanograms and elevated reflex thresholds in ears with type C tympanograms (25).
4.4.3. Cleft Palate Association
A notable 40% (60 out of 150) of children with EA had cleft palate lesions, which were significantly associated with conductive hearing loss. This finding underscores the importance of monitoring middle ear function in children with EA and associated craniofacial anomalies.
The outcomes from audiological testing are summarized in Table 3.
| Test | EA Group Findings; No. (%) | Control Group Findings; No. (%) | Mean Threshold (dB HL) | Curve Type Breakdown | Reflex Threshold (dB) | P-Value |
|---|---|---|---|---|---|---|
| ABR (conductive hearing loss) | 62 (41.3) | 5 (3.3) | 25 ± 5 | - | - | ≤ 0.01 b |
| Tympanometry (middle ear pressure) | 62 (41 type B, 21 type C) | 0 (0 type B/C) | - | Type B = 41 effusion; type C = 21 neg. pressure | - | N/A |
| Acoustic reflex | Absent in 41 (type B); elevated in 21 (type C) | Present in all ears | - | - | Absent = 0 dB; elevated = 85 - 95 dB | N/A |
| Cleft palate presence | 60 (40.0) | - | - | All associated with conductive loss | - | N/A |
Comparison of Audiological Test Results Between Esophageal Atresia and Control Groups a
5. Discussion
Children with EA and TEF often experience long-term complications beyond surgical repair, including esophageal motility disorders, gastroesophageal reflux (3, 26, 27), and potential auditory impairments. Despite advancements in surgical and medical management, the impact of EA on auditory function remains underexplored. This study aimed to evaluate middle ear pathology and its consequences on hearing development in children with EA. Our findings indicate a statistically significant increase in conductive hearing loss among children with EA, as demonstrated by ABR testing (P ≤ 0.01). This aligns with previous studies suggesting that anatomical and physiological factors associated with EA predispose children to recurrent otitis media, eustachian tube dysfunction, and subsequent conductive hearing loss (28).
Notably, 40% of the study population presented with cleft palate, a known risk factor for middle ear effusions and eustachian tube dysfunction, further compounding the risk of hearing impairment. Tympanometric assessments revealed a predominance of type B tympanograms, consistent with persistent middle ear effusion, while type C tympanograms indicated varying degrees of eustachian tube dysfunction (24). Acoustic reflexes were frequently absent or elevated, supporting the presence of conductive pathology (25). These findings reinforce the well-documented association between EA and middle ear dysfunction, warranting early audiological monitoring and intervention.
Several mechanisms may contribute to the observed hearing loss in children with EA. One hypothesis suggests that refluxed esophageal mucus and microorganisms may enter the middle ear via the eustachian tube, leading to chronic inflammation and infection, a mechanism similar to that seen in gastroesophageal reflux-related otitis media (8). Additionally, the horizontal feeding position commonly used for infants with EA may facilitate the movement of secretions into the middle ear, exacerbating the risk of recurrent otitis media (29). Furthermore, the high prevalence of cleft palate among children with EA (reported up to 25% in some studies) suggests that structural abnormalities play a critical role in the development of conductive hearing loss (10). The cleft palate impairs normal eustachian tube function, leading to negative middle ear pressure, effusions, and increased susceptibility to infections (30).
The strong association between EA and conductive hearing loss underscores the importance of early and regular audiological screening in this population. The ABR and DPOAE testing offer objective and reliable methods for detecting hearing impairments, even in neonates, ensuring timely intervention (12-15, 17-19, 31). Given the potential impact on speech and language development (32), routine audiological assessments should be integrated into the long-term follow-up of EA patients. When indicated, ventilation tube placement may help manage persistent middle ear effusions and prevent further auditory complications (33, 34).
To further enhance our understanding of the audiological implications of EA, future research should explore several key areas. One important direction is the investigation of long-term hearing outcomes in children with EA, focusing on how surgical interventions may influence auditory function over time. Additionally, studies should aim to differentiate the impact of anatomical abnormalities, infectious complications, and inflammatory processes in the development of conductive hearing loss in this population. Another crucial area of research involves evaluating preventive and therapeutic strategies, such as the potential benefits of prophylactic antibiotic use or modified feeding techniques, in reducing middle ear infections and subsequent hearing loss.
Despite the valuable insights provided by this study, certain limitations should be acknowledged. The small sample size may restrict the generalizability of the findings to a broader population. Furthermore, the lack of longitudinal follow-up prevents a comprehensive understanding of how hearing status evolves over time in individuals with EA. Additionally, potential biases, such as referral bias in audiological evaluations, should be considered when interpreting the results. Future studies with larger cohorts and long-term follow-up are necessary to address these limitations and provide a more comprehensive perspective on the auditory challenges faced by children with EA.
Although this retrospective design inherently carries risks of referral and selection bias, several methodological steps were taken to mitigate these limitations. First, a census-based sampling approach was used, including all patients diagnosed with EA during the study period, regardless of auditory symptoms, thus reducing the likelihood of selection bias. Additionally, the audiological assessments (ABR and DPOAE) were performed uniformly for all included patients, minimizing information bias. Furthermore, patients were selected based on diagnosis and not referral for hearing loss, helping to reduce referral bias. Despite these efforts, some residual bias may persist due to the retrospective nature of the study and its setting in a tertiary care hospital.
5.1. Conclusions
This study highlights the high prevalence of conductive hearing loss in children with EA and underscores the critical need for comprehensive audiological assessments. The frequent co-occurrence of cleft palate further emphasizes the importance of a multidisciplinary approach, involving audiologists, otolaryngologists, and pediatric surgeons. By implementing routine auditory screenings and early interventions, clinicians can improve speech, language, and overall developmental outcomes for children with EA. Further research is needed to refine management strategies and optimize long-term care for this population.