1. Introduction
Chylopericardium, known as an infrequent complication of cardiovascular surgery, has a reported incidence rate of less than 0.5% in all cardiac surgeries across age groups and occurs rarely following intrapericardial procedures (1).
Some studies have noted that this rare entity in the pediatric population may be due to tuberculosis, malignancy, or congenital lymphangiomatosis (2, 3).
Chylopericardium may also occur following congenital cardiac and thoracic surgery. Both conservative and aggressive treatment strategies are recognized approaches. There is some evidence indicating that these methods yield effective outcomes, although those patients were complicated with only chylopericardium following cardiac or thoracic surgeries (4, 5).
On the other hand, delayed treatment can lead to fatal consequences, and some disputes still exist regarding the etiologies, routes of definitive diagnosis, treatment modalities based on the latest evidence, and preventive measures (6). Additionally, no definitive conclusions can be drawn due to the scattered nature of existing data (7).
Since the coexistence of chylopericardium with novel coronavirus infection may pose a challenge to therapeutic approaches (8, 9), we report the first successful management of chylopericardium complicated by COVID-19 in a pediatric patient following Glenn shunt surgery.
2. Case Presentation
A six-month-old boy weighing 7 kilograms was brought to the emergency department with respiratory distress and a sustained intermittent cough lasting two weeks, which had worsened over the past three days. He was delivered vaginally at 37 weeks of gestational age with a birth weight of 3.1 kilograms. His medical history and echocardiography performed after birth revealed tricuspid atresia type 1B and a single ventricle, for which Glenn shunt surgery was performed two months prior to admission. It is noteworthy that during his hospitalization following cardiac surgery, he exhibited normal pulmonary pressure, without any evidence of pericardial effusion or chylothorax.
Upon arrival, physical examination revealed a heart rate of 120 beats/min, blood pressure of 82/24 mmHg, tachypnea with a respiratory rate of 60 breaths/min, and an SpO2 of 85% measured via a finger pulse oximeter on the index finger. He was in a febrile state with a temperature of 37.9℃.
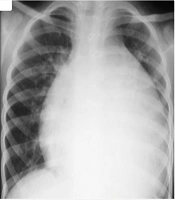
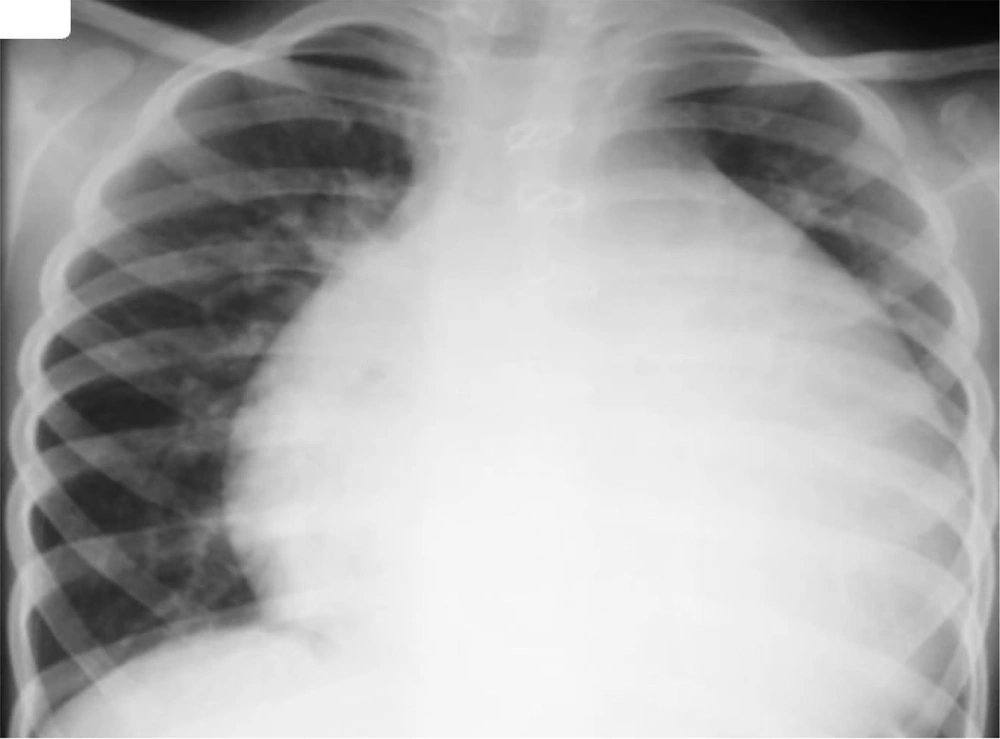
He presented with muffled heart sounds, and the chest X-ray showed cardiomegaly (Figure 1). Accordingly, consultations with pediatric cardiology and cardiac surgery were requested. Echocardiography revealed a structurally normal heart consistent with the recent Glenn shunt surgery but showed a massive pericardial effusion with features of early cardiac tamponade.
Laboratory tests at admission revealed WBC = 4.5 × 103/μL, with lymphocytes accounting for 70%. ESR and hs-CRP levels were within normal ranges. Urine and blood cultures were obtained, and no growth was observed after 48 hours. A viral panel, including RT-PCR tests for COVID-19 and influenza, was performed. The RT-PCR for COVID-19 returned positive.
Based on these findings, emergent needle pericardiocentesis was performed, and 230 mL of milky white serous fluid was drained. Gram stain and acid-fast bacillus (AFB) stain of the drained fluid were negative. The laboratory results from the chylous drainage and the serological assay are illustrated in Table 1. Additionally, the serological analysis of the fluid showed WBC = 3.84 × 103/μL with 5% mononuclear cells, suggesting chylopericardium. Liver and renal function tests were within normal limits.
| Laboratory Tests (Chyle Drained) | Results | Laboratory Tests (Serologic Assay) | Results |
|---|---|---|---|
| Triglyceride | 2000 mg/dL | Triglyceride | 140 mg/dL |
| Cholesterol | 11 gr/dL | Cholesterol | 242 mg/dL |
| Protein | 11,600 mg/dL | LDL | 144 mg/dL |
Some serologic assays revealed that immunoglobulin G (IgG = 512 mg/dL) and immunoglobulin A (IgA = 22.4 mg/dL) were within normal ranges. However, immunoglobulin M (IgM = 168 mg/dL) was elevated in this pediatric patient with COVID-19. Additionally, evaluation of complement components showed normal results for both C3 (402 mg/dL) and C4 (75 mg/dL).
Simultaneously, universal precautions due to COVID-19 were implemented, and antiviral treatment was initiated. The patient was also managed conservatively with fluids and partial parenteral nutrition. Enteral nutrition was initially withheld; this approach continued for 72 hours. After three days, octreotide was initiated at 1 mcg/kg/h, and furosemide infusion was administered at 0.2 - 0.5 mg/h.
On day five, despite ongoing octreotide infusion, the patient continued to produce 50 - 70 mL of chylous fluid per day. Therefore, albumin administration was initiated.
On day six, the pericardial drain was removed due to the transition to serous drainage and a negligible amount of fluid following pericardiocentesis. The octreotide infusion was tapered to 0.5 mcg/kg/h and was subsequently discontinued. Albumin infusion was also stopped on day six, while the furosemide infusion continued until day seven.
Ten days after admission, the patient was discharged from the ICU and monitored closely in an isolated room in the pediatric ward. After two additional days, he was discharged with favorable outcomes and stable hemodynamic parameters.
He was followed up regularly for 18 months after discharge, with serial echocardiography and periodic chest X-rays. Fortunately, there was no recurrence of chylopericardium during the follow-up period, and he demonstrated appropriate growth without any significant complications.
3. Discussion
Chylopericardium is a rare manifestation of pericardial effusion in pediatrics following cardiac surgery, with complex underlying causes (2). Its symptoms also vary depending on the extent of involvement in children and may manifest as dyspnea or shortness of breath, cyanosis, and muffled heart sounds (4).
The clinical expression is generally categorized into two main presentations: First, it may present as cardiac tamponade due to the subclinical accumulation of chyle in the pericardium. Second, it may manifest as an initially serous and milky fluid draining through the thoracic drain (6).
Paraclinical findings supporting the diagnosis of chylopericardium include elevated triglyceride levels in the drained fluid, ranging from 110 to 2000 mg/dL, and confirmation through a cholesterol-to-triglyceride ratio of less than one. These laboratory findings were evident in our patient (1, 7).
Several treatment approaches have been established for chylopericardium. The initial treatment is typically conservative. In our case, we initiated treatment with dietary restriction using medium-chain triglycerides (MCTs) (8). The primary aim of this approach is to reduce lymph production. Additionally, the absorption of these nutrients via the portal system allows the lymphatics to be bypassed (9).
Some current evidence also supports medical management using sympathomimetic drugs. In our patient, an octreotide infusion was administered. This agent accelerates smooth muscle contraction, thereby enhancing lymphatic drainage (10). Although the optimal duration of octreotide administration remains debated and varies across cases (11), we continued its use until a significant decrease in chyle drainage volume was observed.
In some cases, surgical intervention such as pericardiectomy with the creation of a pericardioperitoneal window has been considered for persistent chylous drainage (2). However, this procedure was not necessary in our patient, due to successful management through dietary and medical measures. Meanwhile, it is important to note that conservative management of chylopericardium can be challenging and may sometimes result in treatment failure (1, 3).
We presented the first successfully conservatively managed case of chylopericardium without the need for a significant surgical intervention, such as the establishment of a pericardioperitoneal window.
This outcome may be attributed to the etiology of chylopericardium in our patient. He had undergone Glenn shunt surgery two months prior to admission, which is considered one of the traumatic causes of chylopericardial tamponade. Additionally, this condition was complicated by a concurrent novel coronavirus infection.
It is worth noting that viral infections can lead to life-threatening pericardial effusions (12).
Moreover, several studies have reported that patients with congenital heart defects (CHDs), particularly those with uncorrected CHD (13) and cyanotic defects (14), may be at an increased risk of developing severe COVID-19 (15).
Furthermore, COVID-19 may contribute to the development of chylopericardium by triggering inflammatory pathways or through direct viral involvement of the lymphatic system (16). Therefore, the concurrence of CHD and COVID-19 infection in this case created a highly challenging clinical scenario, significantly impacting decision-making and the overall therapeutic approach.
4. Conclusions
Although the post-operative follow-up period did not reveal any signs of pericardial effusion related to the prior cardiac surgery, the precise cause of chylopericardium in our patient remains unclear. On the other hand, no definitive explanation has been established for the leading etiology in this case. Overall, this highlights a gap in our current understanding. Further investigations are necessary — both in clinical evaluation and paraclinical testing — to clarify the underlying mechanisms and improve diagnostic and treatment strategies for chylopericardium in similar contexts.