1. Introduction
Current literature on mechanical ventilation and weaning in pediatrics is limited. Although several indices and guidelines have been developed to predict successful weaning and extubation, they often fail to produce consistent results. In pediatric clinical practice, clinical judgment is paramount in delivering a tailored regimen to guide gradual weaning and achieve successful liberation from mechanical ventilation. This case report provides a comprehensive overview of managing pediatric acute respiratory distress syndrome (ARDS) complicated by severe scoliosis, an abnormal curvature of the thorax that restricts rib cage expansion. During an acute insult such as pneumonia, the work of breathing and respiratory load increase, potentially leading to respiratory failure in the child.
2. Case Presentation
A 13-year-old girl, measuring 131 cm in height and weighing 25 kg, presented to the emergency department with a 3-day history of fever, cough, and shortness of breath. Upon presentation, she was in severe respiratory distress with a dusky appearance. The child was conscious, sitting upright, and able to speak in sentences of only three to four words, with a blood oxygen saturation of 54% in room air. Her respiratory rate (RR) was 44 breaths per minute, with distinct nasal flaring and intercostal and suprasternal retractions. A non-rebreathing mask at 15 L/min was administered, improving her oxygenation to a blood saturation of 100%. Arterial blood gas (ABG) analysis revealed respiratory acidosis: pH 7.23, PaCO2 94 mmHg, PaO2 87 mmHg, HCO3 38.9 mmol/L, base excess (BE) 7.9 mmol/L, and SpO2 94.3%.
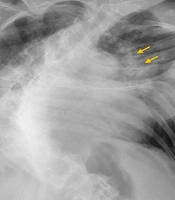
Electrocardiography (ECG) showed a regular sinus rhythm at 175 beats per minute, blood pressure of 155/78 mmHg, and an axillary temperature of 38.4°C. Chest X-ray revealed bilateral diffuse infiltrates, a cardiothoracic ratio of 59%, and thoracolumbar dextroscoliosis with a Cobb angle of 85.3° (Figure 1). Laboratory values and blood cultures were obtained before administering 1 gram of intravenous ceftriaxone. Two hours later, her condition deteriorated; she became more agitated, and her blood saturation fell to 89%. She was sedated with 3 mg of midazolam and intubated with a 6.5 endotracheal tube secured at 16 cm. Mechanical ventilation was initiated using pressure control (PC) mode with a peak inspiratory pressure (PIP) of 15 cm H2O, positive end-expiratory pressure (PEEP) of 8 cm H2O, RR of 20 breaths per minute, and an oxygen fraction (FIO2) of 80%. The tidal volume ranged between 144 and 160 mL, with a total minute ventilation of 3.6 to 4.0 L/min. A repeat ABG showed pH 7.250, PaCO2 85.5 mmHg, PaO2 158 mmHg, HCO3 37.9 mmol/L, BE 10 mmol/L, and SpO2 99%.
Her medical history included arthrogryposis multiplex congenita (AMC), congenital scoliosis, congenital ptosis, and diabetes. Spirometry had not been previously conducted. Her family had been taking her for routine physiotherapy visits and had been advised on corrective surgery at age 10 (Cobb angle 55°) but had electively declined the procedure.
2.1. Progress in the Pediatric Intensive Care Unit
Upon arrival at the pediatric intensive care unit (PICU), the patient’s blood glucose level was 265 mg/dL, and regular insulin was administered at a rate of 2 units per hour. Her glucose level was monitored periodically to maintain a target range of 120 - 180 mg/dL throughout her care. Sedation was achieved using morphine at 1 mg/h, midazolam at 1 mg/h, and dexmedetomidine at 0.2 µg/kg/h. Due to her worsening respiratory status, the antibiotic regimen was adjusted to include intravenous amikacin 400 mg once daily, meropenem 750 mg three times daily, and fluconazole 100 mg twice daily. The ventilator settings were PC with a PIP of 20 cm H2O, PEEP of 8 cm H2O, RR of 30 breaths per minute, and FIO2 of 70%, resulting in a total minute ventilation of 3.5 to 4.1 L/min. Arterial blood gas analysis showed pH 7.282, PaCO2 81.7 mmHg, PaO2 175 mmHg, HCO3 38.6 mmol/L, BE 12 mmol/L, and SpO2 99%. Normal saline was administered at 1000 mL per 24 hours, and oral feeds via a nasogastric tube were initiated at 100 mL four times daily, totaling 500 kcal.
On day 2, the patient experienced hypotension and was administered intravenous norepinephrine at 0.01 µg/kg/min to achieve a targeted mean arterial pressure of 65 mmHg. Ventilator settings remained on PC with a total minute ventilation of 3.6 to 4.0 L/min. Daily ABG results were pH 7.358, PaCO2 64.5 mmHg, PaO2 145 mmHg, HCO3 36.0 mmol/L, BE 11 mmol/L, and SpO2 99%. Sedation continued with morphine at 0.5 mg/h, midazolam at 0.25 mg/h, and dexmedetomidine at 0.2 µg/kg/h, achieving a Ramsay sedation scale of 2; the patient responded to simple commands such as a handshake and mouth opening during oral hygiene.
On day 3, the patient appeared more alert, and a trial of synchronized intermittent mandatory ventilation (SIMV) was attempted with the following settings: Pressure support (PS) 10 cm H2O, PIP 20 cm H2O, PEEP 5 cm H2O, RR 15 breaths per minute, and FIO2 40%. The ABG results revealed pH 7.382, PaCO2 69.4 mmHg, PaO2 79 mmHg, HCO3 41.9 mmol/L, BE 16 mmol/L, and SpO2 99%. Six hours after the trial, the patient began to experience diaphoresis and increasing respiratory distress, prompting a switch back to PC mode with PIP 22 cm H2O, PEEP 5 cm H2O, RR 25 breaths per minute, and FIO2 40%, resulting in a total minute ventilation of 6.0 to 7.0 L/min.
On day 4, ventilatory settings remained at PC with a total minute ventilation of 3.9 to 6.5 L/min, and ABG results were pH 7.486, PaCO2 44.8 mmHg, PaO2 151 mmHg, HCO3 34.1 mmol/L, BE 10 mmol/L, and SpO2 99%. Oral intake was increased to 750 mL per 24 hours, totaling 750 kcal, and normal saline infusion was reduced to 500 mL per 24 hours.
On day 5, the patient remained on the same ventilatory settings due to extreme fatigue observed during routine activities such as mobilization and physiotherapy sessions.
On day 6, the patient appeared more alert, and a trial of adaptive support ventilation (ASV) mode was attempted, set at a body weight of 35 kg, PEEP of 5 cm H2O, and FIO2 of 35%. Within 30 minutes, she appeared to be in distress as her heart rate increased from 70 to 92 beats per minute, and her RR increased from 18 to 42 breaths per minute. Consequently, her ventilator setting was changed to SIMV with PIP of 20 cm H2O, PS of 15 cm H2O, PEEP of 5 cm H2O, rate of 15 breaths per minute, and FIO2 of 35%. Sedation with dexmedetomidine was discontinued. Oral feeding was increased to 1000 kcal daily (1.3 kcal/mL), administered as 5 doses of 150 mL each, and normal saline was reduced to 10 mL/h.
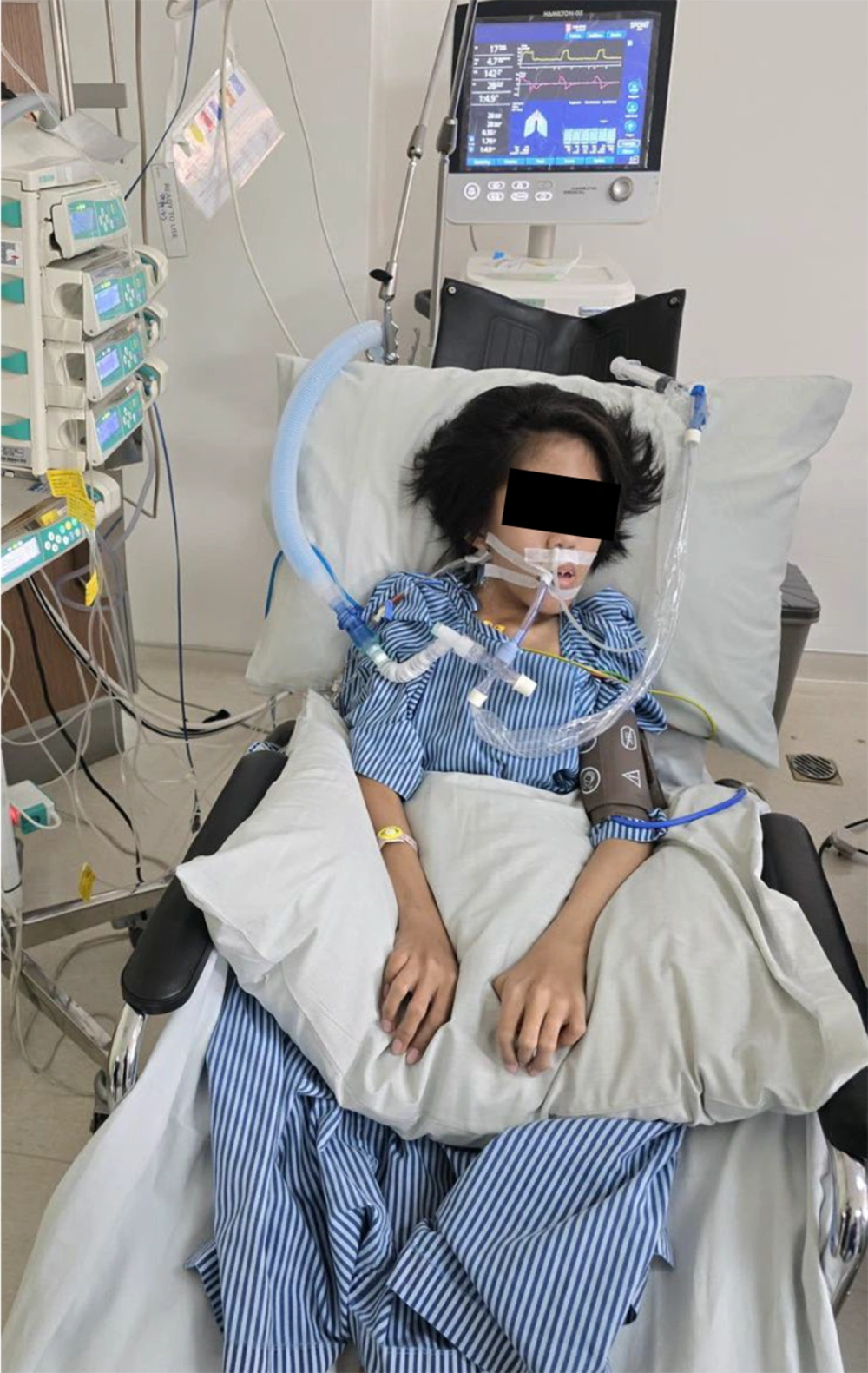
Starting on day 7, norepinephrine was discontinued, and the patient was weaned by sitting in a wheelchair for 60 to 120 min/d (Figure 2). From day 7 through day 9, her ventilatory mode was alternately switched from SIMV to PS mode, set at PS of 15 cm H2O, PEEP of 5 cm H2O, and FIO2 of 35%. A chest X-ray revealed cleared air bronchograms (Figure 3).
On day 10, her ventilatory mode was reduced to PS of 10 cm H2O, PEEP of 5 cm H2O, and FIO2 of 35%. Tidal volume ranged from 126 to 200 mL, with minute ventilation of 3.8 to 6.4 L/min. Morphine infusion was stopped, and she remained calm and tolerant throughout the 24-hour observation period. Her ABG showed pH 7.354, PaCO2 57.4 mmHg, PaO2 117 mmHg, HCO3 32.4 mmol/L, BE 7 mmol/L, and SpO2 98%. She was extubated on day 11, fully alert with good cough reflexes, and transferred to the pediatric ward on day 12. Her recovery was uneventful and she was discharged home on day 19 without any sequel (Table 1).
| Variables | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | Day 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ventilator mode | PC 20/8/30/70% | PC 22/5/24/45% | PSIMV 20/PS 10/5/15/40% alternating with PC 22/5/25/40% | PC 22/5/25/35% | PC 22/5/25/35% | ASV 35 kg 100%/5/35% alternating with PSIMV PC 20/PS 15/5/15/35% | PSIMV 20/PS 15/5/15/35% alternating with PS 15/5/35% | PSIMV 20/PS 15/5/15/35% alternating with PS 15/5/35% | PSIMV 20/PS 15/5/15/35% alternating with PS 10/5/35% | PS 10/5/30% | 2 L/min nasal canule |
| Clinical finding | - | Extreme fatigue during mobilization | Awake and alert | Extreme fatigue during mobilization | Extreme fatigue during mobilization | Awake and alert | Sitted on wheelchair 2 hours/day | Sitted on wheelchair 2 hours/day | Sitted on wheelchair 2 hours/day | Sitted on wheelchair 2 hours/day | Awake and alert |
| ABG | 7.28/81.7/175/38.6/12/99% | 7.36/64.5/145/36/11/99% | 7.38/69.4/79/41.9/16/96% | 7.49/44.8/151/34.1/10/99% | 7.42/49.7/95/32.8/8/98% | 7.53/31.9/145/26.9/4/100% | 7.41/44/123/28/3/99% | - | 7.35/52.3/109/28.8/3/98% | 7.354/57.4/109/32.4/7/98% | 7.38/58.4/107/34.9/10/98% |
| Daily fluid balance | (+) 576 mL | (+) 1022 mL | (-) 563 mL | (+) 290 mL | (+) 7 mL | (+) 381 mL | (+) 384 mL | (+) 337mL | (+) 150 mL | (+) 85 mL | (+) 136 mL |
| Hematology | Hb 12.4/Hct 38.9%/WBC 14.530/Plt 163.000 | - | Hb 10.2/Hct 30% | Hb 11.2/Hct 33% | Hb 11.2/Hct 33% | Hb 10.5/Hct 31% | Hb 10.1/Hct 31.7%/WBC 10.100/Plt 469.000 | - | Hb 10.2/Hct 30% | Hb 10.5/Hct 31% | Hb 11.6/Hct 34% |
| Electrolyte | Na 134 /K 4.5/Cl 94 | - | Na 134/K 4.0/Ca 1.23 | Na 131/K 3.60/Ca 1.18 | Na 130/K 3.60. Ca 1.13 | Na 132/K 3.30/Ca 1.19 | Na 135/K 4.05/Cl 97/Mg 2.30 | - | Na 134/K 3.7/Ca 1.23 | Na 135/K 3.8/Ca 1.25 | Na 134/K 4.4/Ca 1.19 |
| Miscellaneous | - | Bil 0.27, Bil dir 0.10, Bil indir 0.17, Ur 29.8, Cr 0.19 PCT 1.14 | Sterile blood culture | - | - | - | Alb 3.47, Ur 11/Cr 0.14 | - | - | - | - |
Abbreviations: PC, pressure control; ASV, adaptive support ventilation; PSIMV, pressure synchronized intermittent mandatory ventilation; Hb, hemoglobin; Hct, hematocrit; WBC, white blood cell; Plt, platelet; Na, sodium; K, kalium; Cl, chloride; Ca, calcium; PT, prothrombin time; aPTT; activated prothrombin time; Mg, magnesium; Bil, total bilirubin; Bil dir, bilirubin direct; Bil indir, bilirubin indirect; Ur, ureum; Cr, creatinine; PCT, procalcitonin; ABG, arterial blood gas; (+), positive; (-), negative.
3. Discussion
Scoliosis is a chronic condition with significant clinical implications for multiple organs when left untreated. It is categorized into three types: Idiopathic adolescent scoliosis (70%), congenital scoliosis (20%), and neuromuscular scoliosis (20%), with the latter having the worst prognosis due to muscular involvement (1). Early onset (< 8 years), curvatures affecting the thoracic region, and bone immaturity (< 14 years) account for the highest risk of curve progression and organ involvement, all of which are present in our patient. In scoliosis, hypercapnic respiratory failure develops due to the compressive effects of a deformed thoracic cage and impairment in respiratory mechanics (2, 3). Additionally, alveolar flooding with inflammatory cells and leakage of protein-rich pulmonary edema during ARDS causes inactivation of surfactant, leading to alveolar collapse, atelectasis, and hypoxemia (4, 5). Both pathologies contribute to severely reduced pulmonary compliance, leading to type 1 and type 2 respiratory failure, as experienced by our patient. Upon presentation, she had a PaO2/FIO2 (P/F) ratio of 198 and PaCO2 of 86 mmHg. Hence, the initial mode of mechanical ventilation chosen was PC.
Pressure control is a mode of ventilation that delivers mechanical breaths at a set pressure, allowing clinicians to control the distending pressure applied to the airway and subsequently to the alveoli. Higher mean airway pressure in PC results in better outcomes for ARDS patients, as the descending flow rate pattern allows more filling time for alveoli with slower time constants, thus enabling more even aeration between healthy and diseased alveoli (6, 7). The initial setting was a PIP of 20 cm H2O, producing a tidal volume of 6 - 8 mL/kg (pulmonary compliance: 8.75 mL/cm H2O). As her actual RR ranged from 30 to 35 breaths per minute, the mandatory rate was adjusted to 30 breaths per minute with an inspiratory: Expiratory ratio of 1:2. An initial PEEP was set at 8 cm H2O and FIO2 at 70% to improve oxygenation. The following day, a repeat ABG showed improvement in oxygenation (P/F ratio 322), and adjustments to the PEEP level (8 to 5 cm H2O) and FIO2 (70% to 45%) were made accordingly. However, PaCO2 levels remained elevated at 64.5 mmHg.
On day 2, PIP was increased (20 to 22 cm H2O) and RR reduced (30 to 24 breaths per minute) to achieve a larger tidal volume and increase expiratory time. Daily bronchodilator therapy and chest physiotherapy were administered to aid in clearing pulmonary secretions and opening obstructions (3, 8). The following day, she was more awake and alert despite a PaCO2 value of 69.4 mmHg, so a trial of SIMV mode was initiated. Within two hours, she became distressed and was switched back to PC mode. During days 4 and 5, her respiratory mechanics improved, as observed through her chest X-ray, PaCO2 of 44.8 mmHg, and P/F ratio of 431, but she appeared more fatigued, so a weaning trial was not initiated.
In our patient, severe thoracic scoliosis (> 70°) alters the geometry of her thoracic cage and places respiratory muscle function at a disadvantage due to inefficient biomechanics, making basal breathing a difficult task. Conditions such as hypoxia, hypercarbia, and acidosis decrease the efficiency of muscle energy production, while malnutrition decreases muscle mass and oxidative energy-producing enzymes in muscles, predisposing her to early fatigue (9-11). Therefore, providing support to the respiratory muscles through mechanical ventilation and eradicating the infectious process is pivotal to her recovery.
Successful liberation from mechanical ventilation involves balancing the respiratory system by reducing respiratory load and enhancing ventilatory muscle power. Reducing respiratory load requires optimizing pulmonary mechanics and vigorously treating infections. Improving ventilatory muscle power can be achieved through active and passive conditioning (12, 13). An example of passive conditioning is placing the patient in a sitting position, which improves compliance of the respiratory system and chest wall components by changing static lung volumes, enhancing functional residual capacity, and reducing the work of breathing (14). Active conditioning requires the patient’s active participation to gradually increase their fatigue threshold (15). Similar to athletic training of skeletal muscles, ventilatory muscles need conditioning to build endurance and perform adequately. One method of mechanical ventilation weaning is sprint weaning, which involves the abrupt removal of ventilator support during wakefulness approximately 2 to 4 times per day, lasting 1 to 2 minutes. Another method commonly employed in the PICU is SIMV, which involves minimal PS ventilation without disconnection from the ventilator (16). Regardless of the weaning technique chosen, periodic assessment of clinical well-being and adjustments of ventilatory support should be made daily to match the length of stress training until the child can maintain adequate ventilation with minimal support.
Throughout her care, our patient did not receive any neuromuscular blocking agents, as it was imperative for her to maintain ventilatory drive, muscle tone, and cough reflex. Many patients with neuromuscular disorders show sensitivity to non-depolarizing neuromuscular blocking agents, resulting in respiratory weakness, inability to wean, and sputum retention (17). Sedation was administered using continuous drips of morphine, midazolam, and dexmedetomidine, while ventilator asynchrony was promptly managed by adjusting ventilator settings. As her pneumonia resolved, her weaning trial began, and she was placed in a wheelchair with minimal PS ventilation for 60 to 120 minutes daily (Figure 1).
The main limitations of our case report were the unavailability of plethysmography and esophageal pressure monitoring to provide objective evidence of her respiratory muscle function. Without previous spirometry data, we lacked information on the patient's baseline pulmonary function. Therefore, the decision to wean ventilatory support was based on her clinical well-being daily. Her progress was slow; however, we observed that she tolerated longer durations of spontaneous breathing and developed strong cough reflexes day by day. By day 10, she tolerated 24 hours of spontaneous breathing with complete resolution of pneumonia and good cough reflexes, leading to extubation the following day.
Another important aspect in the care of critically ill pediatric patients with ARDS is fluid balance. A positive fluid balance is associated with increased morbidity and mortality (18). At 13 years of age, she weighed 25 kg, indicating a daily fluid intake of 1600 mL. In view of her ARDS, her cumulative enteral and parenteral fluid was set at 1500 mL/d (1000 mL parenteral and 500 kcal enteral) for three consecutive days. As her cumulative fluid balance totaled a positive 1032 mL, her daily fluid intake was decreased to 80% (1250 mL/d) by reducing total parenteral fluid to 500 mL/d while increasing her enteral feeding (750 kcal). Providing nutrition through the enteral route is the most physiologic way to preserve gut integrity and avoid inadvertent fluid overload. Therefore, her enteral feeding was increased to 1000 kcal (750 mL/24 h), while parenteral fluids were reduced to 10 mL/h by day 9 (65% of daily fluid requirement) until liberation from mechanical ventilation.
3.1. Conclusions
Mechanical ventilation in patients with uncorrected scoliosis presents a significant challenge. Over time, derangements in thoracic anatomy lead to ventilatory muscle weakness. Inspiratory muscle weakness hinders deep inspiration and causes atelectasis, while expiratory muscle weakness impairs effective coughing, leading to pooling of pulmonary secretions. These factors increase the incidence and severity of pneumonia. Improving the oxygenation and ventilatory status of patients with scoliosis is a meticulous and time-consuming effort that involves aggressive chest physiotherapy, appropriate antibiotic coverage, and ventilatory muscle conditioning. Even then, liberation from mechanical ventilation may take weeks to months, and the child may progress to chronic respiratory failure.
Nevertheless, the care of pediatric patients with respiratory failure must be tailored to the individual. There are no fixed guidelines regarding the type of mode and duration of mechanical ventilation suitable for a particular individual or situation. Moreover, discrepancies between clinical appearances and laboratory parameters are commonly observed in clinical practice. As such, the decision is primarily a clinical one. In addition to assessing RR, rhythm, effort, and efficacy, the actual mechanics of breathing should be systematically evaluated daily. Clinical responsiveness and assessment of respiratory mechanics are crucial to guide adjustments of ventilator settings and monitor weaning tolerance appropriately.