1. Introduction
Pulmonary vein thrombosis (PVT) is an infrequent and underdiagnosed condition in clinical practice, particularly in pediatric populations. Despite its infrequency, it is a critical and potentially life-threatening event (1). The rarity of this phenomenon can be attributed to the lung's extensive network of venous collateral vessels, which typically prevent obstruction. However, certain pathological conditions can disrupt this balance, leading to PVT (2). Diagnosing PVT is often challenging and requires high clinical suspicion alongside advanced diagnostic imaging modalities (1, 3). In this report, we present the case of a pediatric male patient with diffuse alveolar hemorrhage (DAH) who was diagnosed with PVT in the context of a concurrent novel coronavirus (COVID-19) infection.
2. Case Presentation
2.1. Patient History
A 12-year-old male patient weighing 35 kg was admitted to the emergency department with complaints of severe shortness of breath, frequent coughing, and massive hemoptysis. His symptoms had worsened over the 12 hours prior to hospital arrival. During the initial history-taking, the patient reported prolonged exposure to his mother, who had probable seasonal influenza. He described a gradual onset of shortness of breath that abruptly worsened with a crescendo pattern, culminating in massive hemoptysis. His past medical history included anemia; however, he had no prior hospitalizations or medication use.
On admission, the patient's condition was critical. Hemodynamic parameters included a blood pressure of 88/45 mmHg, heart rate of 152 bpm, respiratory rate of 42 breaths/min, and oxygen saturation of 74% at rest as measured by a finger pulse oximeter. He was febrile with a core temperature of 38.9°C.
2.2. Diagnostic Work-up and Management Strategy
Considering his critical condition, the patient was immediately transferred to the intensive care unit (ICU) for close monitoring and advanced care. In the ICU, he was started on high-flow nasal oxygen therapy. Arterial blood gas analysis revealed severe acute respiratory acidosis. Within 20 minutes, his respiratory effort became insufficient. He experienced massive hemoptysis, a decreased level of consciousness, and an inability to clear airway secretions. Based on his deteriorating ABG parameters and clinical instability, invasive mechanical ventilation was initiated.
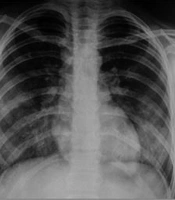
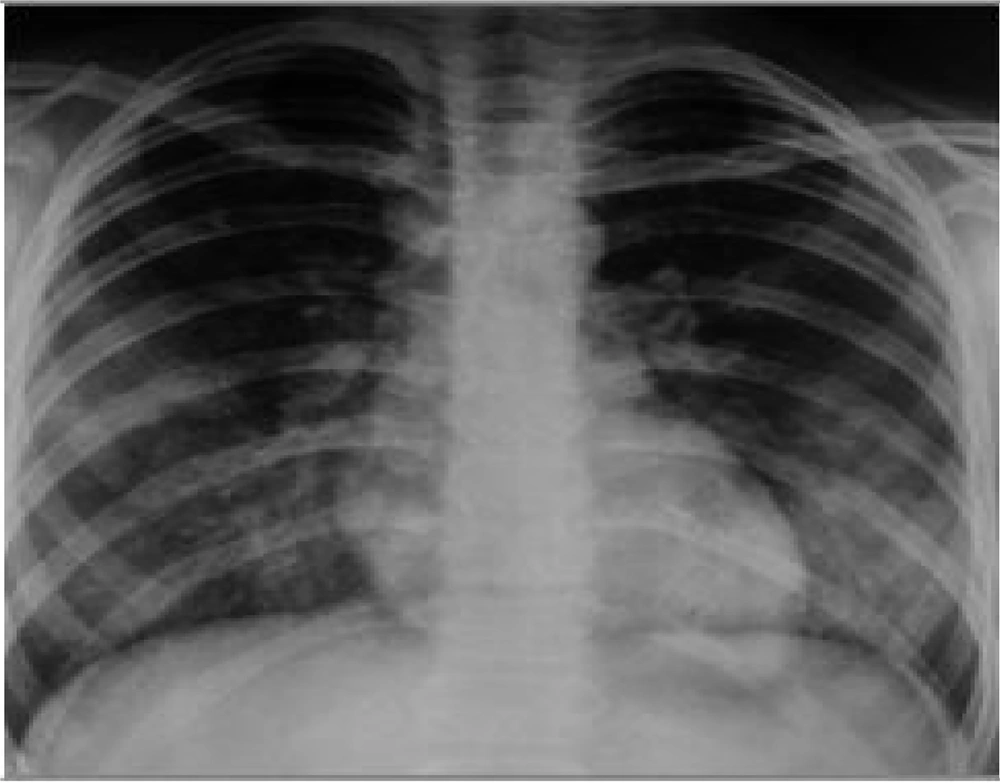
Initial portable chest radiography (anteroposterior view) demonstrated significant bilateral diffuse infiltrates in the upper and lower lobes consistent with pulmonary involvement. However, no evidence of cardiovascular abnormalities or pleural effusion was noted. The costophrenic angles were sharp, with no signs of pleural effusion (Figure 1).
Given the patient's febrile state upon arrival and the exclusion of an infectious etiology, empirical broad-spectrum antibiotics were initiated. He received meropenem (40 mg/kg q8h) and ciprofloxacin (5 mg/kg q12h). Before starting antibiotics, BACTEC blood and urine cultures were obtained, both of which returned negative after 48 hours. A viral panel was also performed for influenza A and B, respiratory syncytial virus, and SARS-CoV-2.
Corticosteroid therapy was initiated after endotracheal intubation with methylprednisolone administered intravenously at 0.5 mg/kg q12h. Laboratory results revealed mild hematuria and glycosuria alongside granular casts in the urine. Procalcitonin levels were elevated (10.21 ng/mL), indicating a high risk of systemic inflammation and organ dysfunction. Hematological parameters on the first day are provided in Table 1.
| Laboratory Test | Result | Laboratory Test | Result |
|---|---|---|---|
| Hemoglobin | 8.5 g/dL | ESR | 34 mm/h |
| Hematocrit | 24.3% | hs-CRP | 119.54 mg/L |
| MCV | 56.8 fl | PT | 11.0 s |
| MCH | 14.9 p.g | PTT | 25.4 s |
| Fibrinogen | 2450 mg/dL | INR | 1.00 |
| FDP | 5 < FDP < 20 | Protein-C | 91 IU/dL |
| D-dimer | 3424 ng/FEU mL | Protein-S | 85 IU/dL |
Abbreviations: MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; FDP, fibrinogen degradation product; ESR, erythrocyte sedimentation rate; PT, prothrombin time; PTT, partial thromboplastin time; INR, international normalized ratio.
D-dimer levels were markedly elevated (3424 ng/FEU mL), prompting a cardiac evaluation. Serologic studies, including the purified protein derivative (PPD) skin test and the Coombs Wright test, returned negative results. Bedside transthoracic echocardiography revealed a preserved left ventricular ejection fraction (about 50% by visual estimation) with no evidence of pulmonary thromboembolism, pericardial effusion, regional wall motion abnormality, or significant valvular heart disease. However, the mean pulmonary artery pressure was elevated (28 mmHg). Electrocardiographic findings showed sinus narrow complex tachycardia without evidence of P-wave abnormalities, heart block, or QTc prolongation.
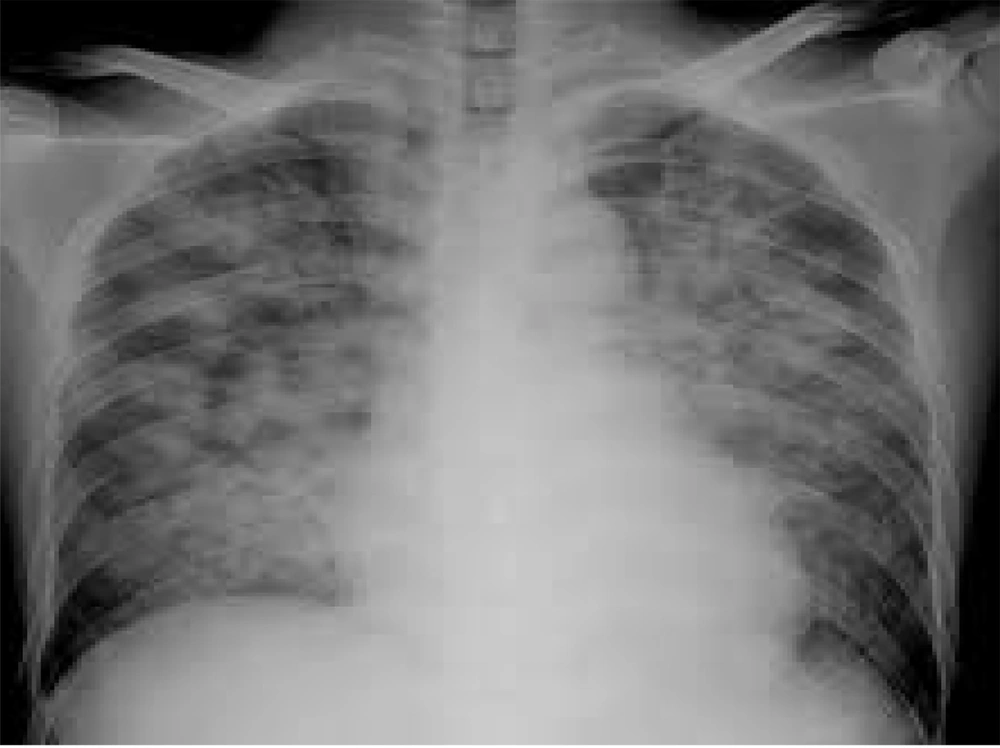
Further autoimmune and inflammatory testing revealed negative results for cytoplasmic antineutrophil cytoplasmic antibodies (C-ANCA), perinuclear antineutrophil cytoplasmic antibodies (P-ANCA), anti-cyclic citrullinated peptide (anti-CCP), antinuclear antibodies (ANA), and the native double-stranded DNA antibody (anti-dsDNA). The patient underwent high-resolution computed tomography (HRCT) of the chest without contrast, which demonstrated significant ground-glass opacities, bronchiectasis, and interlobular septal thickening, particularly in the bilateral upper lobes, consistent with pulmonary hemorrhage (Figure 2). No pleural effusion or mediastinal lymphadenopathy was found.
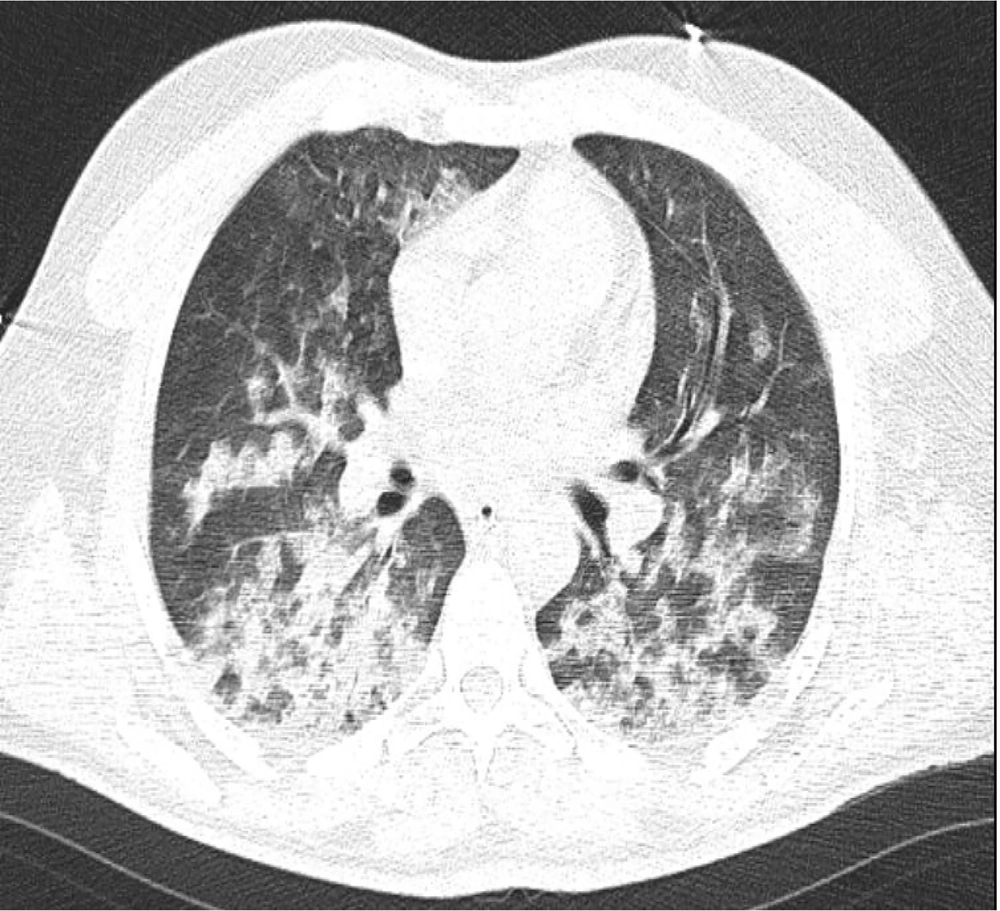
Subsequent contrast-enhanced chest CT revealed a thrombus in the left superior pulmonary vein (Figure 3). The coagulation profile was within normal limits. Considering these findings, enoxaparin (4000 mg, twice daily) was initially prescribed to minimize the risk of embolization. This approach was followed by warfarin (2.5 mg daily) for systemic anticoagulation. Coagulation parameters were monitored daily to balance the risks of anticoagulation therapy and ongoing diffuse alveolar hemorrhage.
On the second day of ICU admission, the patient tested positive for COVID-19. Standard isolation precautions and a COVID-19 management protocol were implemented, including supportive care and medication. Fourteen days after mechanical ventilation, improvement in clinical and ABG parameters allowed the development of a weaning plan. The following day, the patient was successfully weaned from mechanical ventilation, and extubation was performed after 18 hours.
Subsequently, bedside fiberoptic bronchoscopy assessed the alveolar status post-hemorrhage for potential tracheal microbial infection. Bronchoalveolar lavage (BAL) samples revealed hemosiderosis deposition in alveoli consistent with diffuse alveolar hemorrhage. No bacterial, fungal, viral, or pneumocystis carinii infections were identified. Corticosteroid therapy was tapered, and antibiotics were gradually discontinued.
2.3. Follow-up Treatment and Care Strategies
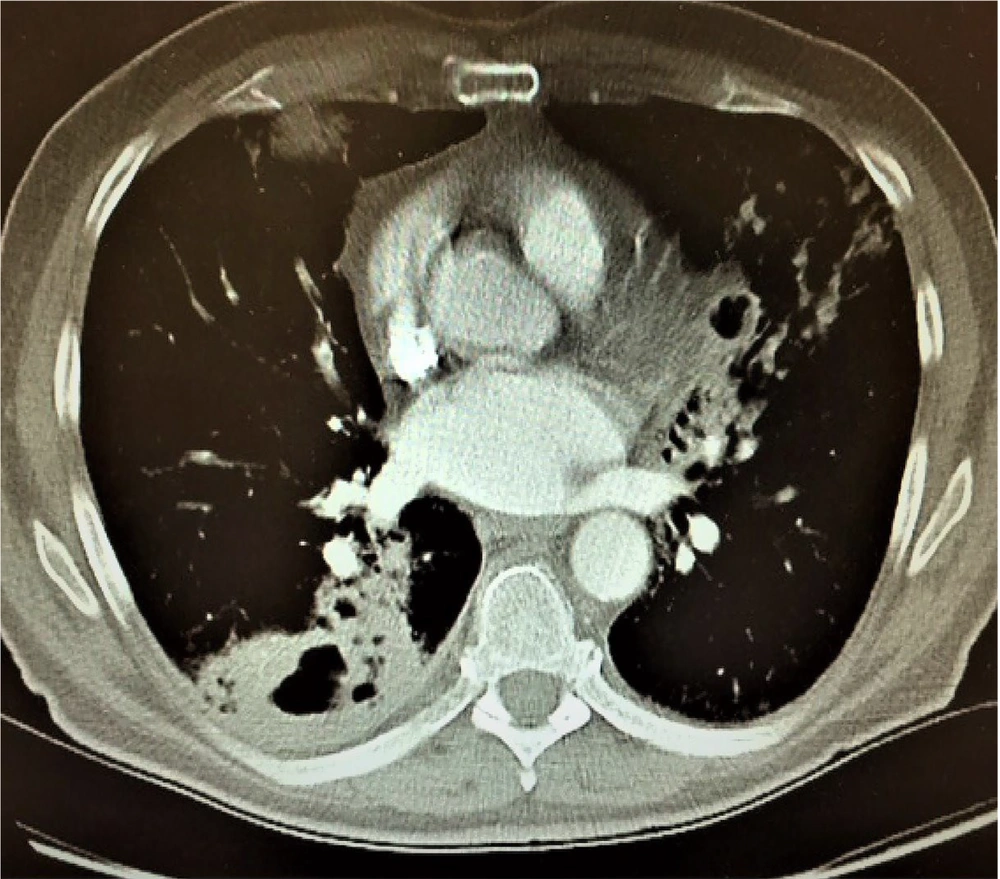
A follow-up chest X-ray showed significant improvement with a reduction in the bilateral opacities (Figure 4). Oxygen therapy was continued via a simple face mask at 8 L/min, later transitioned to a nasal cannula delivering 4 L/min. The patient demonstrated steady clinical improvement and was transferred to the post-ICU in stable condition. Four days later, he was discharged with appropriate advice to address his anemia and avoid exposure to pulmonary irritants.
Warfarin was reduced to 1.25 mg daily, guided by PT and INR measurements performed weekly after discharge. A follow-up echocardiography appointment was recommended three months post-discharge to evaluate cardiac function, particularly pulmonary artery pressure.
3. Discussion
Pulmonary vein thrombosis is an extremely rare condition most often associated with significant pulmonary or cardiac events such as major pulmonary surgery (e.g., transplantation), lung malignancies, chest trauma, arteriovenous malformations, mitral stenosis, radiofrequency catheter ablation for atrial fibrillation, sclerosing mediastinitis, and atrial myxoma (4-6). However, in rare instances, no underlying cause is identified (3). In our patient's case, there was no history of comorbidities or prior hospitalizations, adding to the complexity of the diagnosis.
The PVT can be asymptomatic or present with nonspecific symptoms, including pleuritic chest pain, cough, fever, shortness of breath, and hemoptysis. It is not uncommon for PVT to be misdiagnosed as a pulmonary embolism due to overlapping clinical presentations. Additionally, in our case DAH, a severe and potentially life-threatening condition that results from injury to the pulmonary microcirculation, further confounded the diagnosis. The DAH is characterized by hemoptysis, anemia, diffuse lung infiltrates, and acute respiratory failure (7, 8). The simultaneous presence of PVT and DAH in this patient made it challenging to determine whether DAH or PVT was the primary cause of specific symptoms, as both conditions can share similar clinical manifestations.
The underlying pathophysiology of symptoms in PVT likely involves elevated pulmonary venous pressure, which may lead to secondary increases in pulmonary arterial pressure (3). In this case, echocardiography revealed elevated mean pulmonary arterial pressure (MPAP), which supported PVT as a contributing factor to the patient's symptoms. Previous studies have reported normal pulmonary artery pressures in patients with DAH (7), further suggesting that PVT played a pivotal role in the clinical manifestations observed in this patient.
Laboratory findings in our patient revealed a normal coagulation state, as supported by normal protein C and protein S levels, ruling out deficiencies as the cause of thrombosis. Other studies have highlighted the importance of hypercoagulable states, such as protein C and protein S deficiencies, in developing thrombotic events, including PVT (9-11). While these deficiencies can generally be excluded with normal test results, additional factors may have contributed to thrombus formation (12). One possible explanation is inflammation-mediated platelet activation, which, combined with endothelial damage, may lead to fibrin deposition and thrombus formation (13). Viral infections, including COVID-19, have well-established links to coagulopathy and alterations in the hemostatic balance, resulting in both hemorrhagic and thrombotic complications (14). For example, the influenza virus has been shown to induce platelet aggregation, pulmonary microvascular thrombosis, endothelial dysfunction, and an exaggerated inflammatory response (15, 16). Similarly, the so-called "cytokine storm", triggered by severe COVID-19, is a major driver of acute respiratory distress and pulmonary complications (17). Probably, the diffuse alveolar hemorrhage and subsequent PVT in our patient were precipitated by the proinflammatory and prothrombotic effects of COVID-19.
Diagnosing PVT is notably challenging due to its nonspecific symptoms. Accurate identification often requires a combination of advanced imaging modalities, such as HRCT with contrast, transthoracic echocardiography (TTE), transesophageal echocardiography (TEE), or magnetic resonance imaging (MRI) (18-20). Our patient's HRCT with intravenous contrast proved instrumental in identifying the thrombus within the left superior pulmonary vein. The delayed phase of contrast imaging was beneficial in reducing motion-related artifacts. Transthoracic echocardiography revealed elevated MPAP but lacked other specific findings. Based on the latest guidelines from the American Society of TEE, it was not performed in this case (21).
Management of PVT should be tailored to the underlying pathology and often includes antibiotics (for infectious causes), anticoagulation, or surgical intervention (1). In our patient, empirical antibiotic therapy with meropenem and ciprofloxacin was initially administered to prevent secondary lung infections despite the absence of an identifiable bacterial source. Anticoagulation therapy with low molecular weight heparin (enoxaparin) was initiated to minimize the risk of embolization, later transitioning to warfarin for long-term systemic anticoagulation to dissolve the thrombus. This approach was selected in light of the patient's massive hemoptysis, aiming to reduce hemorrhagic recurrence while managing thrombotic complications. Similar protocols for managing DAH with careful anticoagulation have demonstrated successful outcomes in other studies (10).
The co-occurrence of COVID-19 in this patient added significant complexity to the clinical course, as the hyperinflammatory state compounded the risks of both hemorrhagic and thrombotic complications. This case underscores the importance of a multidisciplinary approach for early recognition, accurate diagnosis, and precise, aggressive treatment of PVT. Managing patients with coexisting conditions like COVID-19 requires meticulous attention and a holistic strategy to optimize outcomes.
In conclusion, the interplay between COVID-19, diffuse alveolar hemorrhage, and PVT presents unique diagnostic and therapeutic challenges. Early and accurate identification of rare conditions like PVT, guided by advanced imaging and a multidisciplinary care strategy, is paramount to reducing morbidity and mortality in such complex cases.