1. Background
Idiopathic nephrotic syndrome (NS) is a significant nephrotic disorder in medical science. Lesions of the basement membrane and increased glomerular permeability in this condition lead to massive protein loss through urine and various clinical manifestations. Nephrotic syndrome is characterized by proteinuria greater than 40 mg/m2/hr or a protein-to-creatinine (Pr/Cr) ratio above 2. Other cardinal features include hypoalbuminemia [albumin (Alb) less than 0.3 g/dL], hypercholesterolemia (Cho above 250 mg/dL), and edema (1). The estimated incidence of idiopathic NS is 2 to 7 cases per 100,000 children under 14 years of age (2). Immune system dysfunction plays a crucial role in the pathogenesis of NS. Components of the complement system, interstitial inflammation, and factors related to blood permeability contribute to disease progression, with the notable response of most patients to immunosuppressive therapy supporting the role of immune-mediated mechanisms (3).
NS is broadly classified based on the underlying cause into two main categories: Primary NS, which includes subtypes such as minimal change nephrotic syndrome (MCNS), focal segmental glomerulosclerosis (FSGS), membranoproliferative glomerulonephritis (MPGN), membranous nephropathy, and congenital forms; and secondary NS, which results from various other underlying conditions such as lupus, Henoch-Schonlein purpura, Wegener’s granulomatosis, and other vasculitides (2). This condition has multiple complications associated with its prognosis (3). Due to hyperlipidemia, proteinuria, and the side effects of immunosuppressive drugs, children with NS are at high risk for developing cardiovascular disease (CVD), including atherosclerosis and hypertension. However, in children, atherosclerosis is often subclinical and predominantly manifests as endothelial dysfunction (4).
Hyperlipidemia in NS is caused by disturbed lipoprotein metabolism, mainly due to increased synthesis and decreased catabolism. This leads to elevated levels of total Cho, triglycerides, very-low-density lipoprotein (VLDL), and low-density lipoprotein (LDL), often referred to as "bad" Cho. Dyslipidemia further enhances the risk of developing atherosclerosis and CVD (5). Analysis of blood samples from pediatric NS patients demonstrated significant abnormalities in their lipid profile. Total Cho, LDL Cho, triglycerides, and endothelin-1 (ET1) were significantly higher, while high-density lipoprotein (HDL) Cho, Alb, and paraoxonase 1 (PON1) levels were much lower compared to a control group (6, 7).
Other important characteristics of NS include hypercoagulability, due to increased urinary loss of antithrombin III, altered protein C and S activity, enhanced fibrinogen synthesis by the liver, and increased platelet aggregation (8, 9). This predisposes patients to recurrent thrombotic and embolic events. While venous thrombosis is more common in adults, arterial thrombosis predominates in children, which can present with complications such as renal vein thrombosis, pulmonary embolism, stroke, and myocardial infarction (10). Carotid intima-media thickness (CIMT) is a reliable marker for assessing medium and large blood vessel atherosclerosis, with increased CIMT and endothelial dysfunction often preceding the onset of CVDs (5, 11).
Research also suggests that renal dysfunction in children with NS increases atrial natriuretic peptide concentrations, a marker of high CVD risk (12). Hypertension, another common complication, can cause heart failure and cardiomyopathy, where acute hypertension aggravates the outcome. The three types of cardiomyopathies — dilated, hypertrophic, and restrictive — may culminate in bradycardia, ventricular fibrillation (VF), asystole, and sudden death (13).
2. Objectives
While there is no strong evidence yet to support routine heart screening for children with NS, regular heart checkups could help detect silent heart issues early, before any symptoms appear. Early detection can lead to earlier treatment and better long-term health outcomes. Since the effects of NS on the heart in children are not completely understood and no clear guidelines are in place, many experts are exploring the benefits of using echocardiography early in the disease. Including this simple, non-invasive test in regular care could help caregivers monitor the heart more closely, act quickly when needed, and reduce the risk of serious complications. According to the aforementioned materials, this study aimed to evaluate echocardiography parameters in children with NS and compare them with healthy children.
3. Methods
This case-control study involved all children with NS referred to the Clinic and Pediatric Nephrology Department of Ali Ibn Abi Talib Hospital (AS) from 2021 to 2023. Nephrotic syndrome was identified by the criteria of proteinuria > 40 mg/m2/hr, hypoalbuminemia (less than 3 g/dL), hypoproteinemia (less than 5.5 g/dL), hypercholesterolemia (higher than 250 mg/dL), and peripheral edema. Inclusion criteria for the study participants included age less than 18 years, absence of congenital kidney disease, agreement to participate in the study, and meeting the criteria for NS. Exclusion criteria were severe heart, liver, or kidney diseases, and types of secondary NSs.
3.1. Study Implementation
Patients diagnosed with idiopathic NS according to the diagnostic criteria or previously treated for NS were recruited for this study after being informed about the research project. After blood sampling, their information was recorded on the NS patients’ information form, and they were referred to a pediatric cardiologist for echocardiography for cardiac examinations. Following echocardiography, their echocardiographic findings were documented in the relevant form.
The control group subjects were selected from children who visited the nephrologist or cardiologists for routine annual checkups, matched for age and gender with the children with NS, without underlying disease and without a history of NS in the patient or their family. Data were recorded on the healthy children’s information form after informed consent was obtained from their parents or the patients themselves. These subjects were also referred to the pediatric cardiologist for cardiac examinations requiring echocardiography, and their echocardiographic findings were documented.
During this period, entry was made considering the exclusion criteria of the study for all children with NS, and an equal number of healthy children were collected as controls.
3.2. Laboratory Measurements
A 2 mL sample of venous blood was collected in a plain tube and allowed to clot in an incubator. Once clotted, the sample was centrifuged to separate the serum. Biochemical tests were conducted to measure serum Alb, total Cho, urea, and creatinine (Cr) levels using spectrophotometry with the Abbott Architect C-8000 system (Abbott Diagnostics, Santa Clara, CA).
3.3. Echocardiographic Assessments
Each participant underwent a standard echocardiographic evaluation, including M-mode and two-dimensional imaging, performed by a cardiologist using the My Lab 60 system with 3 - 8 MHz transducers (manufactured in Italy). Several key heart function parameters were measured, including the thickness of the left ventricular posterior wall during systole (PWDS) and diastole (PWDD), the Myocardial Performance Index (MPI), and the ratio of early to late mitral valve flow velocity (E/A ratio). Additional measurements included ejection fraction (EF), fractional shortening (FS), interventricular septal thickness during diastole (IVSDD) and systole (IVSDS), as well as left ventricular diastolic dimension (LVDD) and left ventricular end-systolic (LVSD) dimensions.
To evaluate global myocardial function, the MPI, also known as the Tei Index, was calculated using the formula: MPI = (a - b)/b , where "a" represents the interval between the end and beginning of mitral or tricuspid inflow (i.e., total systolic-diastolic time excluding ejection), and "b" is the ventricular ejection time (ET). To obtain "a", the pulsed Doppler sample volume was placed at the tips of the mitral and tricuspid valve leaflets in the apical four-chamber view, allowing measurement of the total time interval from the end of one inflow to the start of the next. The sample volume was then repositioned in the apical five-chamber view, just below the aortic valve, to measure "b", the left ventricular ET. For right ventricular assessment, the outflow velocity pattern was recorded in the parasternal short-axis view, with the Doppler sample volume placed just distal to the pulmonary valve. Each parameter was measured over three consecutive cardiac cycles, and the average value was used to ensure accuracy and minimize variability.
3.4. Ethical Approval
Before the study began, informed consent was obtained from all participants or their parents. The research was reviewed and approved by the Ethics Committee of Zahedan University of Medical Sciences, under the Children and Adolescent Health Research Center (IR.ZAUMS.REC.1402.007).
3.5. Data Analysis
After data collection, the information was entered into SPSS version 23. Mean and standard deviation were used to describe quantitative data, while frequency and percentage were used for qualitative data. After checking for normality, different parametric and non-parametric tests, such as the t-test and Mann-Whitney U test, were applied to compare variables between the two groups. The chi-square statistical test was used to compare categorical data. For all statistical analyses, a significance level of P < 0.05 was considered.
4. Results
A total of 174 subjects were enrolled in the study, comprising 87 patients with NS and 87 healthy controls. The case group had a higher percentage of male subjects compared to the control group. However, there was no significant difference in age between the two groups. Notably, the case group exhibited significantly lower weight compared to the control group.
Table 1 presented the levels of Cr, Cho, Alb, and the Pr/Cr ratio in children with NS. Regarding Cr, the mean value was 0.78 ± 0.86 mg/dL. The Cho levels ranged from a minimum of 117 mg/dL to a maximum of 650 mg/dL, with a mean of 324.13 ± 106.12 mg/dL. Albumin ranged from 1.20 to 4.00 g/dL, with a mean of 2.20 ± 0.55 g/dL. The Pr/Cr ratio ranged from 0.26 to 23.16, averaging 5.09 ± 4.38 mg/mmol/L. These values provide insights into the clinical status of the participants.
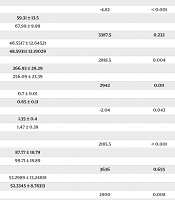
Table 2 showed the echocardiographic findings, which consisted of conventional and Doppler echocardiography for both the left and right ventricles. The table revealed significant impairment in left ventricular functions in NS patients, manifested by changes in the following parameters: Left ventricular systolic dimension (P < 0.001), EF (P < 0.001), and FS (P < 0.001). Significant changes were also observed in right ventricular functions in NS patients compared with the controls. The significant parameters included a decrease in peak E velocity (P < 0.001), an increase in ET (P = 0.004), an increase in the MPI (P = 0.011), and a decrease in the E/A ratio (P = 0.043). Similar results were noted for left ventricular functions in NS patients, with significant parameters being a decrease in peak E velocity (P < 0.001), a decrease in ET (P = 0.008), an increase in MPI (P = 0.002), and a decrease in the E/A ratio (P < 0.001).
Table 3 presented the correlation coefficients (r) and their corresponding P-values between different biochemical parameters, including Cho, Cr, Alb, and the Pr/Cr ratio, and echocardiographic parameters in both conventional and tissue Doppler. The analysis indicated that no significant correlations were found between biochemical parameters (serum Alb, Cr, Cho, and urine protein-creatinine ratio) and echocardiographic parameters. Therefore, these factors might not be major determinants of cardiac dysfunction in the population studied.
| Variables | Range | Mean ± SD |
|---|---|---|
| Cr | 0.30 - 8.00 | 0.78 ± 0.86 |
| Cho | 117.00 - 650.00 | 324.13 ± 106.12 |
| Alb | 1.20 - 4.00 | 2.20 ± 0.55 |
| Pr/Cr ratio | 0.26 - 23.16 | 5.09 ± 4.38 |
Laboratory Findings in Patients with Nephrotic Syndrome
| Measures and Groups | Mean ± SD | Test Value | P-Value |
|---|---|---|---|
| Conventional echocardiography | |||
| LVDD | -1.69 | 0.093 | |
| Case | 3.61 ± 0.52 | ||
| Control | 3.76 ± 0.59 | ||
| LVDS | 7.611 | < 0.001 | |
| Case | 2.48 ± 0.39 | ||
| Control | 2.07 ± 0.32 | ||
| EF | 59.5 | < 0.001 | |
| Case | 0.61 ± 0.05 | ||
| Control | 0.77 ± 0.05 | ||
| FS | 16.5 | < 0.001 | |
| Case | 0.31 ± 0.03 | ||
| Control | 0.41 ± 0.03 | ||
| Doppler right ventricular | |||
| E | -4.82 | < 0.001 | |
| Case | 59.31 ± 13.5 | ||
| Control | 67.99 ± 9.99 | ||
| A | 3387.5 | 0.232 | |
| Case | 46.5517 ± 12.64521 | ||
| Control | 48.5931± 12.19029 | ||
| ET | 2818.5 | 0.004 | |
| Case | 266.93 ± 29.29 | ||
| Control | 256.09 ± 23.39 | ||
| MPI | 2942 | 0.011 | |
| Case | 0.7 ± 0.01 | ||
| Control | 0.65 ± 0.11 | ||
| E/A | -2.04 | 0.043 | |
| Case | 1.35 ± 0.4 | ||
| Control | 1.47 ± 0.38 | ||
| Doppler left ventricular | |||
| E | 2185.5 | < 0.001 | |
| Case | 87.77 ± 18.79 | ||
| Control | 99.71 ± 18.89 | ||
| A | 3636 | 0.655 | |
| Case | 53.2989 ± 13.24818 | ||
| Control | 52.3345 ± 8.78313 | ||
| ET | 2900 | 0.008 | |
| Case | 256.95 ± 25.49 | ||
| Control | 267.2 ± 19.48 | ||
| MPI | 2732 | 0.002 | |
| Case | 0.74 ± 0.11 | ||
| Control | 0.65 ± 0.19 | ||
| E/A | -3.55 | < 0.001 | |
| Case | 1.71 ± 0.41 | ||
| Control | 1.94 ± 0.45 |
Echocardiographic Parameters: Comparison Between Case and Control Groups
| Echocardiography Parameters | Cho | Cr | Alb | Pr/Cr | ||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| LVDD | -0.06 | 0.576 | 0.01 | 0.918 | -0.06 | 0.596 | 0.1 | 0.344 |
| LVDS | -0.03 | 0.78 | -0.008 | 0.944 | -0.07 | 0.501 | 0.12 | 0.291 |
| EF | 0.01 | 0.919 | 0.07 | 0.524 | 0.06 | 0.59 | -0.008 | 0.939 |
| FS | -0.04 | 0.69 | 0.1 | 0.354 | 0.06 | 0.614 | -0.03 | 0.798 |
| ET right | -0.041 | 0.708 | 0.003 | 0.975 | 0.012 | 0.909 | 0.05 | 0.643 |
| MPI right | 0.088 | 0.417 | 0.024 | 0.823 | -0.153 | 0.157 | 0.016 | 0.882 |
| E right | -0.092 | 0.395 | 0.033 | 0.759 | 0.113 | 0.298 | 0.167 | 0.121 |
| A right | 0.098 | 0.367 | -0.135 | 0.211 | 0.096 | 0.377 | -0.07 | 0.518 |
| E/A right | -0.133 | 0.219 | 0.163 | 0.132 | 0.022 | 0.84 | 0.158 | 0.143 |
| ET left | -0.077 | 0.476 | 0.025 | 0.718 | 0.066 | 0.541 | 0.214 | 0.134 |
| MPI left | 0.1 | 0.365 | -0.1 | 0.36 | 0.01 | 0.911 | -0.08 | 0.448 |
| E left | 0.005 | 0.966 | -0.1 | 0.344 | -0.06 | 0.561 | 0.03 | 0.812 |
| A left | -0.12 | 0.271 | -0.02 | 0.822 | 0.05 | 0.64 | -0.06 | 0.578 |
| E/A left | 0.11 | 0.301 | -0.06 | 0.561 | -0.05 | 0.666 | 0.14 | 0.193 |
Correlation Matrix: Biochemical Parameters and Echocardiographic Parameters in Children with Nephrotic Syndrome
5. Discussion
The findings of this study indicate significant impairments in both right and left ventricular diastolic functions among children with NS. However, correlation analysis revealed no significant associations between biochemical parameters and cardiac function, suggesting the involvement of other underlying mechanisms in the observed cardiac dysfunction. Systemic complications associated with childhood NS, including cardiovascular, infectious, hormonal, and renal complications, have been well-documented. Hilmanto et al. (14) highlighted that such complications seriously affect the health of affected children. Kamel et al. (15) illustrated the role of tissue Doppler echocardiography in evaluating cardiac dysfunction in patients with NS. Unlike conventional echocardiography, tissue Doppler revealed significant diastolic dysfunction, represented by prolonged relaxation times, shortened early filling times, and elevated MPI. They showed that high MPI values correlate significantly with disease severity and, therefore, with disease duration, frequency of relapses, steroid resistance, and use of immunosuppressive therapy.
Similarly, in the study by Al Mamun et al. (16), cardiac abnormalities, especially left ventricular hypertrophy, were highly prevalent in children with advanced stages of chronic kidney disease (CKD). These abnormalities were associated with anemia, hyperparathyroidism, and increased phosphate, which increase the workload on the heart. Despite this, systolic function remained relatively preserved, indicating compensatory mechanisms. Saleh et al. (17) reported that NS significantly impairs both left and right ventricular diastolic functions, even in the absence of overt systolic dysfunction. The study demonstrated increased diastolic blood pressure, reduced serum Alb, and elevated urinary protein excretion in NS patients compared to controls. These changes were characterized by increased E/A ratios, prolonged isovolumic relaxation times (IVRT), and reduced early diastolic filling times.
Nalcacioglu et al. (13) reported significant cardiac abnormalities in children with idiopathic NS during active disease and remission phases. In these patients, there was impaired left ventricular diastolic function along with systemic changes like higher Body Mass Index, blood pressure, and proteinuria with reduced serum Alb levels. El-Gamasy and El-Shehaby (18) noted a reduced EF in 23.3% of patients with NS, whereas AbdelMassih et al. (19) reported significant disturbances in both systolic and diastolic left ventricular function in children with newly diagnosed NS. AbdelMassih et al. (19) underscored hypoalbuminemia as a major determinant of myocardial dysfunction and observed that steroid therapy has an independent effect on myocardial function regardless of Alb levels. Ashoor et al. (20) further reported that children with primary glomerular diseases, such as NS, have a high prevalence of modifiable cardiovascular risk factors, especially untreated dyslipidemia.
Collectively, research by authors such as Nalcacioglu et al. (13) and Kamel et al. (15) has focused on cardiac function assessments, while studies by Hilmanto et al. (14) and Ashoor et al. (20) addressed broader systemic complications and cardiovascular risks. These studies emphasize that routine cardiac review and timely management can help reduce long-term cardiovascular complications in children with NS. The current study showed that NS patients had high Cho levels (324.13 ± 106.12 mg/dL), low Alb (2.20 ± 0.55 g/dL), and a high Pr/Cr ratio (5.09 ± 4.38 mg/mmol/L). These findings were similar to those reported by AbdelMassih et al. (19). However, no significant correlations between biochemical markers (Alb, Cho, Cr, and Pr/Cr ratio) and echocardiographic parameters were found in the current study. This divergence may be due to differences in study populations or methodologies.
The observed impairments in systolic function, including decreased LVDD, EF, and FS, are consistent with findings by El-Gamasy and El-Shehaby (18). Additionally, this study expands on previous findings by examining more comprehensive systolic parameters. Diastolic dysfunction, indicated by reduced E wave, increased E/A ratio, prolonged IVRT, and increased MPI, mirrors the findings of AbdelMassih et al. (19), who reported similar impairments in NS patients. However, unlike AbdelMassih et al. (19), no significant correlation was found between serum Alb levels and myocardial function in the present study, suggesting the need to investigate alternative mechanisms, such as inflammation or genetic predispositions.
Routine cardiac evaluations, including tissue Doppler echocardiography, are strongly recommended for early detection and management of cardiac dysfunction in children with NS. The increasing reliance on both conventional and Doppler echocardiography reflects the multifaceted impact of NS on cardiovascular health. Conventional echocardiography is essential for assessing global cardiac parameters, while Doppler techniques provide insights into diastolic function and right ventricular involvement, which are critical for timely intervention. The findings of the present study, alongside those of Kamel et al. (15) and Al Mamun et al. (16), emphasize the importance of echocardiographic monitoring in guiding clinical decisions and optimizing outcomes for children with NS.
The findings of the present study also indicate significant impairments in both right and left ventricular diastolic functions among children with NS. However, correlation analysis revealed no significant associations between biochemical parameters and cardiac function, suggesting the involvement of other underlying mechanisms in the observed cardiac dysfunction. Kamel et al. (15) demonstrated the utility of tissue Doppler echocardiography in assessing cardiac function in NS patients. Unlike conventional echocardiography, tissue Doppler identified significant diastolic dysfunction, including prolonged relaxation times, reduced early filling times, and increased MPI.
One of the primary strengths of this study lies in its comprehensive assessment of cardiac functions using both conventional and Doppler echocardiography, which provided a more sensitive and nuanced evaluation of myocardial performance in children with NS. By examining a wide range of echocardiographic parameters, including diastolic indices, MPI, and systolic function measures, this study offers a holistic view of cardiac involvement in pediatric NS. Moreover, the inclusion of a control group allowed for meaningful comparisons, enhancing the internal validity of the findings.
Despite these strengths, the study is not without limitations. First, the cross-sectional design precludes any inference of causality or temporal relationships between NS and cardiac dysfunction. Longitudinal studies are needed to evaluate the progression and clinical implications of cardiac involvement over time. Second, the sample size was relatively small, which may limit the generalizability of the findings to broader populations. Finally, the study did not stratify patients based on disease duration, frequency of relapses, or treatment regimens, which could have influenced cardiac outcomes.
5.1. Conclusions
This study demonstrates that children with NS exhibit significant cardiac dysfunction, including impaired left and right ventricular systolic and diastolic performance, despite the absence of strong correlations with traditional biochemical markers such as Cho, Alb, Cr, and the Pr/Cr ratio. These findings suggest that NS may contribute to subclinical cardiac abnormalities through mechanisms independent of conventional metabolic disturbances. Early echocardiographic assessment should be considered in NS patients to detect potential cardiac dysfunction, even in the absence of overt symptoms. Further research is needed to elucidate the underlying pathophysiology linking NS to cardiovascular impairment.