1. Background
COVID-19 is a novel virus, and research on its infectivity and pathogenesis in the pediatric population is still evolving. In a meta-analysis of 1,810 participants, approximately 25% of the infections were observed in children aged 6 to 10 years old (1). During the first peak of the pandemic in England, approximately 2.8% of the children tested positive for COVID-19. Furthermore, studies have indicated that among children under 5 years old, approximately 53% of infections were observed in infants younger than 1 year old (2, 3). The signs and symptoms of COVID-19 vary based on different age groups (4). Neonates diagnosed with COVID-19 are categorized based on whether they exhibit symptoms. Full-term infants born to infected mothers typically remain asymptomatic or present with mild, nonspecific signs such as unstable body temperature, episodes of apnea, breathing difficulties, gastrointestinal disturbances, and low blood pressure. In preterm neonates, symptoms may also be vague, including respiratory and cardiovascular complications such as rapid breathing, grunting, nasal flaring, labored breathing, apnea, coughing, elevated heart rate, and impaired feeding. Additionally, digestive issues such as vomiting, diarrhea, and abdominal swelling may occur (5).
It remains unclear whether the clinical findings in newborns are a result of direct infection or complications arising from maternal infection (6). It is reported that maternal COVID-19 infection significantly affects the birth weight and gestational age of neonates, despite negative COVID-19 PCR results (7). Furthermore, it is important to note that PCR tests for COVID-19 in neonates may produce false negatives. This can occur for several reasons, such as testing conducted during the early stages of infection when the viral load is low, inadequate sample collection, or errors in the testing process (6). Therefore, more research and careful interpretation of test results are necessary to accurately assess the COVID-19 status in neonates. It is important to note that a negative PCR test result means that the virus was not detected at the time the sample was collected and tested, but it does not guarantee that the person is not infected (8). In cases where neonates present with symptoms but their PCR tests come back negative, additional diagnostic tests may be considered. These can include serological antibody tests or repeat PCR testing (9).
2. Objectives
In this study, we aim to introduce a population of neonates who experience signs and symptoms of infection while testing negative for COVID-19 by PCR, despite having direct exposure to the virus within their families, as confirmed by PCR testing. Understanding the false-negative PCR results in infants is crucial for accurate diagnosis and management, especially given the high transmission potential of COVID-19 in this population.
3. Methods
The participants in this study were infants less than 60 days old who presented with clinical symptoms of infection, including cough, diarrhea, respiratory distress, poor feeding, tachypnea, tachycardia, vomiting, and fever, and were admitted to the NICU or neonatal ward of the Children's Medical Center Hospital. Importantly, all the neonates included in the study had negative PCR COVID-19 tests despite having an index case positive in their family.
3.1. Inclusion and Exclusion Criteria
All infants admitted to the NICU and neonatal ward with symptoms of infection and a history of direct exposure to a COVID-19-positive family member, as confirmed by PCR testing, were included in the study. Importantly, despite their exposure and symptoms, all neonates were required to have negative COVID-19 PCR results at least twice in our center within 24 hours. Neonates with positive COVID-19 PCR, bacterial or fungal infections, and those without parental consent to participate and publication were excluded from the study.
3.2. Exclusion of Confounding Cases in the Study
The study implemented several methodological approaches to exclude confounding factors and ensure the validity of its findings.
3.2.1. Strict COVID-19 Testing Criteria
All neonates included in the study had to test negative for SARS-CoV-2 at least twice via RT-PCR, despite having been exposed to a COVID-19-positive family member. This ensured that any observed symptoms were not due to active COVID-19 infection, reducing misclassification bias.
3.2.2. Exclusion of Other Infections
Neonates with confirmed bacterial or fungal infections were excluded from the study. This eliminated cases where symptoms were attributable to other infectious agents rather than the effects of exposure to SARS-CoV-2.
3.2.3. Standardized Inclusion Criteria
Only neonates with infection-like symptoms (e.g., cough, diarrhea, respiratory distress, fever) and a documented history of direct exposure to a COVID-19-positive family member were included. This ensured that the sample population had consistent exposure history, controlling for variability in external transmission risks.
3.2.4. Parental Consent Requirements
Neonates whose parents did not provide consent were excluded from the study, maintaining ethical research practices. This prevented discrepancies in data collection and ensured adherence to standardized monitoring protocols.
By enforcing these inclusion and exclusion criteria, the study effectively minimized confounding factors, allowing for a more precise evaluation of neonatal symptoms.
3.3. Sample Size
All neonates who met two major criteria — presence of infection symptoms and a history of direct exposure to a COVID-19-positive family member — and were admitted to the NICU and neonatal ward between August 2020 and August 2021 were included in this study.
3.4. Study Design
This observational cross-sectional study was conducted in the NICU and neonatal ward of the Children's Medical Center Hospital in Tehran, Iran, between August 2020 and 2021. RT-PCR samples with Pishtaz Teb's (98001) kit were taken from the neonates’ nasopharyngeal swabs in our center, and the test was repeated after 24 hours. A combination of demographic data, clinical symptoms, laboratory data, radiological findings if indicated, and outcomes based on a pre- arranged questionnaire were collected. Patient files, nursing sheets, and interviews with parents were utilized for collecting data. This project was conducted with the approval and supervision of the Ethics Committee of Tehran University of Medical Sciences, with a license number IR.TUMS.CHMC.REC.1400.166. All data included in this study were collected from patient files in the archives, and patient confidentiality was strictly maintained.
3.5. Statistics
Statistical analysis was performed using the statistical package for the social sciences (SPSS) software version 26 (SPSS Inc., Chicago, USA). Continuous variables were compared using an one sample t-test. The data are presented as mean ± standard deviation (SD) for continuous variables, and a P-value < 0.05 was considered statistically significant.
4. Results
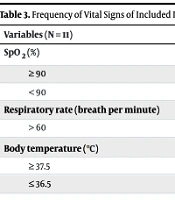
After excluding confounding cases, a total of 11 neonates who were admitted with symptoms of viral infection and had negative PCR results were included in this study. All of them had at least one family member with a positive COVID-19 PCR. Among them, there were 3 girls and 8 boys. Three of the neonates were born prematurely. All cases had late-onset sepsis. The average hospital stay was 7.18 days, with a minimum stay of 3 days and a maximum stay of 15 days (Table 1). The mean onset of symptoms in our patients was 2.73 days, with a minimum of 1 day and a maximum of 5 days. The most common chief complaints during the first visit included respiratory distress, cough, and poor feeding. Respiratory distress and cough were reported by 63.6% and 45.5% of the patients, respectively, while poor feeding was reported by 45.5%. Among the patients, 27.3% experienced NEC (necrotizing enterocolitis) (Table 2). Infants with gastrointestinal symptoms tended to be younger, while those with respiratory symptoms were older. However, these differences were not statistically significant (P > 0.05). The patients with respiratory distress required free-flow oxygen or non-invasive ventilation. None of the patients required invasive ventilation, and there were no mortalities among the infants. The frequency of vital signs during the admission of included infants is shown in Table 3.
| Variables (N = 11) | N (%) | Median (IQR) |
|---|---|---|
| Sex (male) | 8 (66.6) | - |
| Gestational age (wk) | - | 39 (36, 40) |
| Birth weight (g) | - | 3163 (3000, 3200) |
| Age of admission (d) | - | 22 (6, 36) |
| Time of hospitalization (d) | - | 7.18 (3, 15) |
| Index cases | ||
| Mother | 7 (63.6 ) | - |
| Father | 3 (27.3) | - |
| Sibling | 9 (82) | - |
| Variables (N = 11) | N (%) |
|---|---|
| Cough | 5 (45.5) |
| Cyanosis | 2 (18) |
| Diarreha | 3 (27.3) |
| Fever | 4 (36.4) |
| Necrotizing enterocolitis | 3 (27.3) |
| Poor feeding | 5 (45.5) |
| Respiratory distress syndrome | 7 (63.6 ) |
| Seizure | 1 (9) |
| Variables (N = 11) | N (%) |
|---|---|
| SpO2 (%) | |
| ≥ 90 | 9 (82) |
| < 90 | 2 (18) |
| Respiratory rate (breath per minute) | |
| > 60 | 7 (63.6) |
| Body temperature (℃) | |
| ≥ 37.5 | 4 (36.4) |
| ≤ 36.5 | 2 (18) |
Leukopenia and leukocytosis were not observed among the patients. Only one case showed mild neutropenia, with a count of 1160 µL. In approximately 90.9% of the cases, there was a significant increase in lymphocyte count, with a maximum value of 6210. Just one patient had a high CRP (48 mg/dL), and two neonates had thrombocytosis, who had the longest hospitalization periods (11 and 15 days) (Table 4). All cases were discharged with a good early outcome.
| Variables (N = 11) | Median (IQR) |
|---|---|
| C-Reactive protein (mg/dl) | 5.5 (1, 48) |
| Platelets (µ/L) | 335500 (280250, 580000) |
| WBC ( µ/L) | 9300 (8300, 11500) |
| Hb (g/dL) | 14 (9, 15) |
| Lymphocyte count (µ/L) | 4930 (4110, 6210) |
| Neutrophil count (µ/L) | 3270 (1160, 4200) |
5. Discussion
In this cross-sectional study, we aimed to observe a group of neonates showing signs and symptoms of infection, who tested negative for the COVID-19 PCR but had direct exposure to the virus. Doddaiah et al., in a comparative study, showed that maternal COVID-19 infection significantly affects the birth weight and gestational age of neonates, despite negative neonatal PCR results (7). Zhu et al. have recommended assessing the clinical manifestations in neonates born to COVID-19-positive mothers, irrespective of their negative COVID-19 test results, as false-negative test results are common in infants (10). The positivity rate of RT-PCR was found to be higher in females compared to males, unlike our study, and there was a noticeable increase in the likelihood of testing positive with age in Nikam et al.'s study (11). Wu et al., using serologic and molecular biologic methods, mentioned that on the first day of symptoms, RT-PCR testing showed positive results in approximately 32% of suspected cases. The positivity rates peaked at 50 - 60% between day 7 and day 10, after which they gradually decreased. However, in some cases, traces of the virus could still be detected up to 38 days after the onset of illness (12). The quantitative RT-PCR analysis of the nasopharyngeal aspirates revealed a V-shaped pattern in which the average geometric viral loads were lowest on day 5, increased on day 10, and then decreased again on day 15 following the onset of symptoms (13). Results from both the Toronto and Hong Kong outbreaks indicated that the highest rates of positive RT-PCR results and highest virus concentrations in patients' specimens were detected around 9 to 11 days after the symptoms first appeared (14). The mean onset of symptoms in our patients was 2.73 days. The high rate of false-negative PCR results in our study could be attributed to low viral load during the early stages of infection. Parents are understandably worried about their baby's risk of COVID-19 infection and the progression of the disease. As a result, they bring their infants to the hospital promptly at the onset of symptoms. The incubation period in newborns is uncertain, which raises concerns about the adequacy of PCR testing for detecting infections in this population. It is important to note that a negative test result does not guarantee the absence of infection in a newborn (15). Therefore, serological testing or repeat PCR testing is required to improve the diagnostic accuracy of detecting infections in newborns (16). Bhuiyan et al. conducted a meta-analysis on COVID-19 cases in children younger than five years. Their study found that 53% of the COVID-19 infections occurred in children who were less than one year old. Additionally, approximately 43% of the infections in infants less than one year old were asymptomatic, while the remaining infants developed mild to moderate symptoms (3). In another case-control study by Li et al., they described that 40% of positive PCR neonates were asymptomatic (17). Despite the presence of asymptomatic COVID-19 positive infants and symptomatic COVID-19 negative infants, it is crucial to exercise caution due to the high transmission potential of the disease from infants (18). It is true that the COVID-19 pandemic has been resolved, but the lack of attention to the possibility of transmission from infants contributes to the re-emergence of the disease.
5.1. Conclusions
Babies have been observed to be susceptible to acquiring and transmitting the COVID-19 disease. Therefore, it is crucial to take necessary precautions to prevent the transmission of infection to others. Other studies with better design and more comprehensive findings are recommended.
5.2. Limitations
This study had some limitations; one of them was the lack of testing for other viruses, which could have contributed to the symptoms observed in our neonates. Additionally, the small sample size may limit the generalizability of our findings. Conducting further studies is necessary to obtain a more accurate diagnosis.