1. Background
Epilepsy, defined as recurrent seizures, is among the most common neurological disorders in children under 15 years of age, affecting approximately 11 million children worldwide (1-3). It significantly contributes to childhood morbidity and mortality and is associated with various complications, including neurodevelopmental, neurobiological, psychological, and behavioral issues. The etiology is multifactorial, encompassing genetic, immunologic, infectious, metabolic, and structural factors (3). Although dietary or surgical treatments may be used in some cases, antiseizure medications (ASMs) are the mainstay of therapy (3-5). Long-term use of ASMs requires careful monitoring due to potential adverse effects.
Childhood is a critical period for bone metabolism and mineralization. Vitamin D deficiency is a substantial health concern in the pediatric population (5). One of the significant side effects of ASMs is vitamin D deficiency, which reduces bone mineral density (BMD) and increases the risk of fractures (6). Children with epilepsy are already at increased risk for falls and fractures (6). Studies show the frequency of falls and fractures in patients using ASMs is 2 - 6 times higher than in the general population (6, 7). Contributing factors include seizure-related falls, postural instability due to ASMs, and their negative impact on bone and vitamin D metabolism. Hypovitaminosis D is common in this group, leading to muscle weakness, falls, and fractures (7, 8).
Vitamin D is a fat-soluble vitamin essential for calcium and phosphorus metabolism. In the brain, it regulates cell production and differentiation, synaptic transmission, and immune function (3). ASMs significantly impact bone health (6-8). They are generally classified into enzyme-inducing drugs including carbamazepine, phenytoin, and topiramate, which accelerate hepatic vitamin D metabolism, and non-inducing drugs including clonazepam, levetiracetam, valproate, and zonisamide. Enzyme inducers exert greater effects on BMD (9, 10). Non-inducers may still promote vitamin D catabolism via CYP3A4 and CYP24A1 enzyme pathways (11). Additional mechanisms contributing to vitamin D deficiency include mutations in vitamin D receptors, reduced vitamin D activity, decreased intestinal calcium absorption, secondary hyperparathyroidism, and downregulation of bone protein synthesis, such as osteonectin and collagen (12, 13).
Several studies report correlations between ASM serum levels and reduced vitamin D in epileptic children (10, 14, 15), while others dispute a direct relationship between long-term ASM use and vitamin D insufficiency (16, 17). Risk factors may include ketogenic diets, younger age, puberty, high BMI, focal seizures, use of enzyme-inducing or older ASMs, and polytherapy (16, 18-20). Hypovitaminosis D may also be influenced by gender, skin pigmentation, air pollution, physical activity, seasonality, urban residence, geographical location, chronic illnesses including skin diseases, malabsorption, cholestasis, and renal insufficiency, and chronic medication use such as ASMs and glucocorticoids (3).
2. Objectives
While many studies have explored the prevalence and determinants of vitamin D insufficiency in children on ASMs, findings have been inconsistent. Therefore, this study aimed to evaluate the prevalence and primary risk factors of vitamin D insufficiency in epileptic children receiving ASMs in Tehran, Iran. To our knowledge, limited studies in Iran have addressed this issue, making this research a valuable addition to the literature.
3. Methods
A cross-sectional study was conducted at the Neurology Ward of Ali Asghar Children’s Hospital in Tehran, Iran, from October 2020 to April 2021. Participants included inpatient and outpatient epileptic children aged 1 to 14 years undergoing ASM therapy. Eligible children were identified during their hospital visits. The study was explained to participants and their parents, and written informed consent was obtained. Exclusion criteria included febrile seizures, metabolic bone disease, severe or chronic renal, hepatic, or endocrine disorders, recent vitamin D supplementation (within 3 months), malnutrition, and special diets such as the ketogenic diet. All children followed a balanced diet.
A 3 mL blood sample was collected from each child, stored at -20°C, and analyzed using the chemiluminescence method (IDS Co., UK). Vitamin D levels > 30 ng/mL were considered sufficient, 20 - 30 ng/mL as insufficient, and < 20 ng/mL as deficient. The latter two were grouped as non-sufficient (21). Calcium (Ca), phosphorus (P), and alkaline phosphatase (ALP) levels were measured spectrophotometrically, and magnesium (Mg) was measured using atomic absorption spectrophotometry.
Demographic and clinical data, including age, gender, BMI, seizure type and frequency, seizure etiology, medication regimen (mono or polytherapy), developmental delays, and duration of treatment, were collected from medical records. Laboratory results were also documented.
3.1. Statistical Analysis
Data analysis was conducted using SPSS v27.0. Fifteen subjects were excluded due to incomplete data or insufficient samples, leaving 45 participants. The normality of vitamin D levels was verified using the Kolmogorov–Smirnov test. Quantitative variables were presented as mean ± standard deviation (SD), and categorical variables as frequencies and percentages. Categorical variables were compared using the chi-square or Fisher's exact test, and continuous variables using the t-test or Mann–Whitney U test. A P-value ≤ 0.05 was considered statistically significant. Multivariable logistic regression was used to identify predictors of vitamin D insufficiency. ROC analysis was employed to evaluate the diagnostic performance of age as a predictor.
3.2. Ethical Considerations
This study was approved by the Ethics Committee of Iran University of Medical Sciences in accordance with the Declaration of Helsinki [ID: IUMS;1142]. Written informed consent was obtained from the parents or guardians of all participants.
4. Results
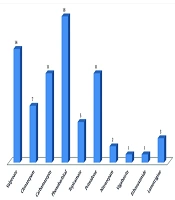
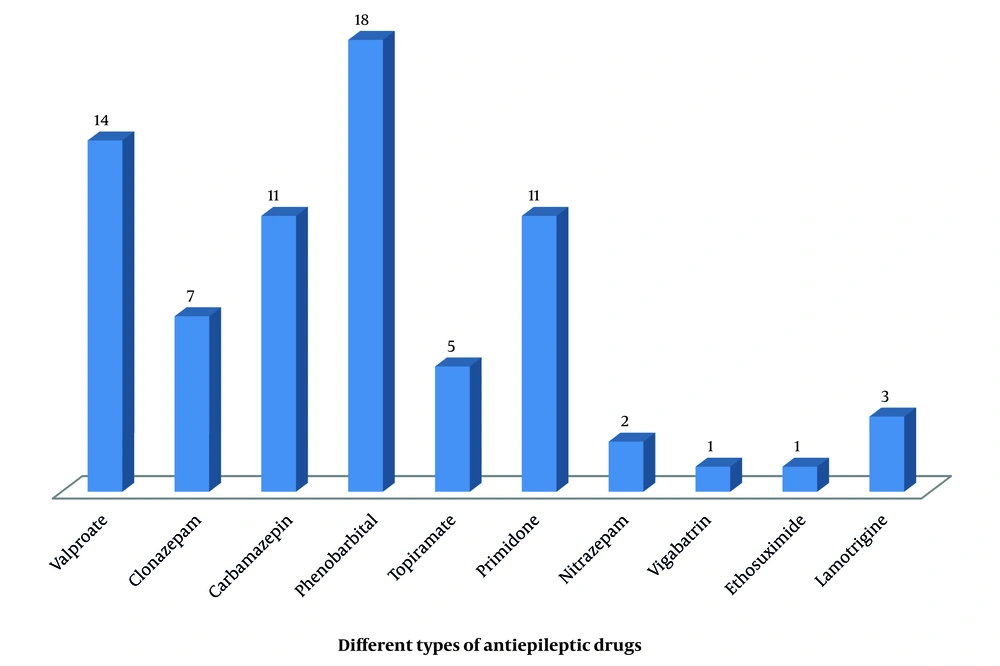
Baseline characteristics of children with epilepsy: Table 1 shows the baseline characteristics of the 45 children who participated in the study. The mean age was 6.31 ± 3.97 years, and 27 (60%) were male. The mean BMI was 16.81 ± 3.39 kg/m2 (range: 12.5 - 30.0). Generalized seizures were observed in 35 (77.8%) patients, while 10 had focal seizures. Etiologically, 23 (51.1%) were symptomatic and 22 (48.9%) idiopathic. Phenobarbital and valproate were the most commonly prescribed drugs. Twenty-six (57.8%) children received monotherapy, while the remainder received combination therapy. Among these, 11 (24.4%) were on two drugs and 8 (17.8%) on three drugs. A majority, 41 (93.3%), were on older-generation ASMs (Figure 1). Seizure frequency varied: About half were seizure-free; 1 (2.2%) had daily seizures; 5 (11.1%) had 1 - 4 per month; and 17 (37.8%) had one per month. The average treatment duration was 2.40 ± 1.14 years. Six children (13.3%) had intellectual disabilities, and 15 (33.3%) had motor developmental delays. The mean serum vitamin D level was 24.18 ± 13.14 ng/mL (range: 7 - 55 ng/mL). In total, 26 children (57.7%) had insufficient or deficient vitamin D levels. Those with insufficient levels were significantly older (P = 0.002) and had longer treatment durations (P = 0.034).
| Characteristics | Insufficient and Deficient Group (n = 26) | Sufficient Group (n = 19) | P-Value b |
|---|---|---|---|
| Age (y) | 7.53 ± 3.96 | 4.12 ± 2.63 | 0.002 |
| Duration of treatment (y) | 2.56 ± 1.12 | 2.05 ± 0.99 | 0.034 |
| BMI (kg/m2) | 16.75 ± 3.18 | 16.12 ± 1.95 | 0.157 |
| Gender (male) | 14 (53.8) | 13 (68.4) | 0.323 |
| Symptomatic seizure | 15 (57.7) | 8 (42.1) | 0.301 |
| Multi-drug treatment | 13 (50.0) | 6 (31.6) | 0.217 |
| Mental retardation | 4 (15.4) | 2 (10.6) | 0.640 |
| Developmental delay | 9 (34.6) | 6 (31.6) | 0.833 |
Comparison of Different Variables Between the Sufficient and Insufficient Groups a
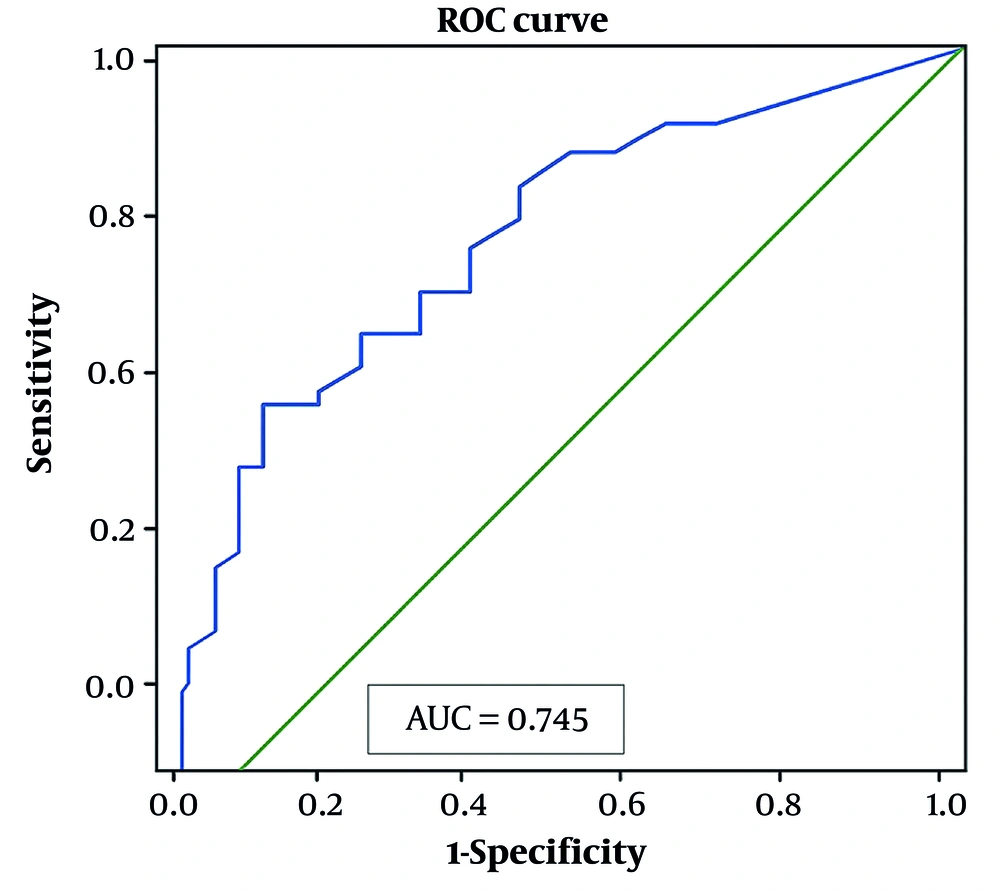
Determinants of vitamin D deficiency: Table 2 shows the binary logistic regression of vitamin D deficiency predictors. No significant associations were found with other factors, including gender, BMI, seizure type, frequency, therapy type, comorbidities, or serum element levels. Multivariate logistic regression did not identify any independent predictors of vitamin D insufficiency. However, ROC analysis indicated that age was a significant predictor, with an AUC of 0.754 (P = 0.043) (Figure 2).
| Variables | P-Value | Odds Ratio | 95% CI |
|---|---|---|---|
| Gender | |||
| Male | 0.397 | 0.583 | 0.168 - 2.030 |
| Female | Reference | ||
| Etiology of seizure | |||
| Primary | 0.223 | 0.626 | 0.620 - 7.300 |
| Secondary | Reference | ||
| Motor developmental delay | |||
| Yes | 0.790 | 0.680 | 0.400 - 11.630 |
| No | Reference | ||
| Mental retardation | |||
| Yes | 0.332 | 3.090 | 0.310 - 30.240 |
| No | Reference | ||
| Developmental delay | |||
| Yes | 0.633 | 1.37 | 0.37 - 5.10 |
| No | Reference | ||
| Calcium level [mg/dL] | |||
| Sufficient | 0.820 | 1.12 | 0.41 - 3.04 |
| Insufficient | Reference | ||
| Magnesium level [mg/dL] | |||
| Sufficient | 0.990 | 0.99 | 0.24 - 3.99 |
| Insufficient | Reference | ||
| Phosphorus level [mg/dL] | |||
| Sufficient | 0.630 | 4.65 | 0.91 - 23.60 |
| Insufficient | Reference | ||
| Alkaline phosphatase level [Iu/L] | |||
| High | 0.264 | 1.02 | 0.99 - 1.00 |
| Low | Reference | ||
| BMI [kg/m2] | |||
| ≥ 25 | 0.466 | 0.92 | 0.72 - 1.61 |
| < 25 | Reference |
Main Determinants of Vitamin D In-sufficiency in Epileptic Children
5. Discussion
This study aimed to determine the prevalence and determinants of vitamin D insufficiency or deficiency among epileptic children treated with ASMs. The study revealed that the prevalence of reduced vitamin D levels (either insufficiency or deficiency) was 26 cases (57.7%), and age was identified as the only significant determinant of vitamin D deficiency among the epileptic children. Although several factors influence vitamin D status, children with epilepsy, particularly those undergoing treatment with ASMs, appear to be at increased risk of hypovitaminosis D.
Studies from various countries (10, 16, 22-24) have evaluated serum vitamin D levels in epileptic children, considering diverse geographic, nutritional, cultural, and lifestyle variables. However, the present study is one of the few investigations (25-27) to assess both the prevalence and significant determinants of vitamin D insufficiency or deficiency in an Iranian pediatric population. The findings indicated that the mean serum vitamin D level among participants was low [24.18 ± 13.14 ng/mL], with a notably high prevalence of insufficiency or deficiency at approximately 58%. This common complication may be attributable to the effects of ASMs, particularly older-generation medications, which stimulate hepatic enzymes or other liver mechanisms, accelerating vitamin D degradation (10, 19).
Other studies from Iran and various Asian countries have similarly reported high prevalence rates of this complication in epileptic patients. For instance, an Iranian study reported that 75% of 89 children undergoing mono- or polytherapy with ASMs exhibited vitamin D insufficiency or deficiency (28). Likewise, Indra Gunawan et al. from Indonesia documented a comparable mean serum vitamin D level of 23.4 ng/mL among 60 epileptic children treated with ASMs for at least six months (17). A study in Egypt found that 62.2% of 45 epileptic children receiving antiepileptic drugs (AEDs) had suboptimal vitamin D levels. Similarly, research in India reported that approximately half of 50 participants (48%) aged 1 to 18 years undergoing mono- or polytherapy had insufficient or deficient vitamin D levels. An investigation from Hong Kong (29) also reported a high prevalence of vitamin D insufficiency, affecting 69% of 71 epileptic children aged 3 - 18 years on ASMs.
In this study, children with insufficient or deficient vitamin D levels tended to be older, with age identified as a significant risk factor for hypovitaminosis D. The higher prevalence of vitamin D insufficiency in older children may be linked to reduced sun exposure due to spending more time indoors, poor dietary habits, conservative clothing styles, or gastrointestinal absorption disorders common in older age groups. These findings are consistent with other studies that have reported a greater incidence of vitamin D insufficiency among adolescents compared to younger children (25). However, some studies have produced conflicting results. For example, Bezboruah and Kalita (30) and Lee et al. (23) found no significant association between age and vitamin D status in patients receiving AED treatment.
Regarding the association between the duration of antiepileptic treatment and vitamin D insufficiency, although a direct relationship was observed in univariate analysis, it was not supported by multivariate regression analysis. This discrepancy might suggest a weak correlation or indicate that longer treatment durations are necessary to significantly impact vitamin D levels. Supporting evidence includes a study from Iran (26), which reported a significant inverse relationship between vitamin D levels and AED therapy duration [P = 0.01; r = -0.345]. A Turkish study (24) similarly identified a significant association between long-term AED use and vitamin D insufficiency in 172 epileptic children. Another investigation (23) found a notable negative correlation between vitamin D levels and treatment periods of two years or more. In contrast, a study from China (16) found a high prevalence (49%) of vitamin D deficiency or insufficiency in 648 epileptic children receiving AED treatment but did not identify any significant correlation between treatment and vitamin D status. The authors suggested that low vitamin D levels may be attributed to the underlying disease rather than the treatment.
This study did not find significant associations between vitamin D levels and other variables such as gender, BMI, seizure etiology, seizure frequency, treatment type, presence of comorbidities, nutritional status, number of prescribed medications, or serum element levels. These findings align with those of Likasitthananon et al. (19), who also reported no significant links between BMI, epilepsy cause, or mono- versus polytherapy and hypovitaminosis D. However, contrary to these findings, Lee et al. (23) noted that tube feeding and BMI might negatively influence vitamin D levels. In summary, this study revealed a high prevalence of vitamin D insufficiency, particularly among older children. A notable limitation is its cross-sectional design, which restricts the ability to determine causal or temporal relationships. Future longitudinal cohort studies are recommended to establish more definitive conclusions.
5.1. Conclusions
The results of this study highlight a high prevalence of vitamin D non-sufficiency among epileptic children on ASMs, particularly in older age groups. Early diagnosis through regular monitoring of serum vitamin D levels, along with timely treatment and prescription of prophylactic vitamin D supplements, should be considered to prevent potential complications. Future investigations with larger sample sizes and the inclusion of more variables are strongly suggested.