1. Background
Measles is a highly infectious disease that may well be eradicated because of its low genetic variation and inability to manifest as a subclinical disease in human beings. The mortality rate associated with measles is highly dependent on the nutritional status of the populaceand may vary from 1% to 30% in different communities.
Patients with measles may show serious complications, such as blindness. The World Health Organization (WHO) reports show that about 30,000,000 new cases of measles occur annually in developing countries and cause 300,000 deaths, accounting for 40% of the deaths caused by vaccine preventable diseases (1). The disease has been eliminated in many western countries by implementing aggressive strategies, including “catch-up” mass campaigns to vaccinate all children aged 1 to 14 years, “mopup” campaigns for children who missed the “catch-up” campaign, efficient routine vaccination services capable of reaching 90% of infants, strong surveillance activities, prompt outbreak response, and “follow-up” campaigns every 24 years that focus on 1-4 year-old children (2).
To control the outbreaks of the disease in our country, all individuals aged 5 to 25 years were revaccinated in anational campaign named “National Measles Rubella (MR) Vaccination Program” from December 22nd 2002 to January 5th 2003.
2. Objectives
The aim of this study was to compare the anti-measles antibody titers between revaccinated and non-revacci- nated individuals 2 years after the national MR vaccina- tion program.
3. Patients and Methods
This study was a stratified cross-sectional study conducted on healthy medical students who attended our hospital in Tehran from summer 2005 to winter 2005 for their pediatric rotation. The revaccinated group was randomly selected from a list of students who had undergone revaccination and the non-revaccinated group included all the non-revaccinated students. Subjects with a history of severe measles or any other infectious disease, fever, immunodeficiency, or any systemic disease such as heart failure or renal impairment were excluded in order to decrease the effect of confounding variables and determination the actual difference in antibody level between two groups.
Based on our assumption 10% of students were protected before Revaccinated, (Non published reports) and protection rate may reach at least 40% after revaccination
and number of cases in each group was calculated approximately 34 cases.
All subjects filled up a questionnaire that documented their age, sex, number of years of education, and history of skin rashes or any infectious disease.
Blood samples (5 mL of peripheral blood) were collected from all participants by their permission. Serum titers of anti-measles IgG and IgM antibodies were measured using an indirect enzyme-linked immunosorbent assay (ELISA) method with the IgG and IgM RF566 11 kit (IBL Company, Hamburg, Germany). After blood sampling, the serum was separated and stored in a refrigerator at -70 ºC; 78 samples were collected. The samples were thawed at room temperature and tested with ELISA at 25 ºC. Each serum sample was diluted to the strength of 1:101 in a standard buffer solution and added to the 4 standard wells A, B, C, and D (e.g. A1, B1, C1, and D1 for specimen number 1). After incubation at 25 ºC for 60 min, the wells were emptied and rinsed thrice with 0.3 mL of buffer, and then the plates were dried by inverting them on an absorbent material. Then, 0.1 mL of secondary conjugated enzyme was added to each well. After a 30-min incubation at 25 ºC, the wells were emptied, rinsed, and dried again as mentioned above. Then, 0.1 mL of Chromogen (TMP) solution was added to each well and after incubation at room temperature for 20 min, 0.1 mL of stopping solution was added and optical density (OD) was measured at 450 nm with an ELISA reader, (model ICN MS212 ) (Tecon) Switzerland. IgG and IgM titers were estimated using a standard curve of logarithmic OD values.
Data were analyzed using chi square test and independent samples t test. Statistical significance was defined as P < 0.05. We used SPSS ver.13 software for data analysis.
There was no other limitations for the study.
4. Results
The study was performed on 78 persons, of which 45 subjects with the mean age of 23.8 ± 0.8 years had been revaccinated. The mean age of the non-revaccinated group was 24.0 ± 1.5 years (P = 0.2). The revaccinated group included 29 women (64.4%) and 16 men, (35.6%) and the non-revaccinated group was composed of 19 women (57.6%) and 14 men (42.4%) (P = 0.544).
There was no intergroup significant difference in the measles vaccination and disease history (P = 0.6 and P = 0.7).
Table 1 shows two baseline variables such as age, gender,number of children in family, mother occupational status,father’s occupation as physician. As it is seen number of children in family is higher in non-revaccinated and occupation of father as a physician in higher in revacci nated group, although these variables have no effect on antibody levels in students.
aAbbreviation: NS, non-significance
bIndependent t test
cChi-square test
The mean anti-measles IgG titer was 31.8 ± 21.2 IU/mL (95% CI = 25.6-38.0), (range, 1-190 IU/mL) in the revaccinated group and 6.12 ± 8.8 IU/mL (95% CI = 3.1-9.1) (range, 1-45 IU/mL) in the non-revaccinated group (P = 0.004). The mean anti-measles IgM titer was 2.6 ± 6.7 IU/mL (95% CI = 0.6-4.6), (range, 1-45 IU/mL) in the revaccinated group and 1.0 ± 0.0 IU/mL (range, 0-1 IU/mL) in the non-revaccinated group (P = 0.1).
The results of anti-measles IgG titer assessments were classified into 3 groups according to the detected levels of the antibody, viz. negative (≤ 7 IU/mL), undetermined (8-12 IU/mL), and positive (≥ 13 IU/mL); 46.7% individuals in the revaccinated group and 6% in the non-revaccinated group were IgG positive (P < 0.001).
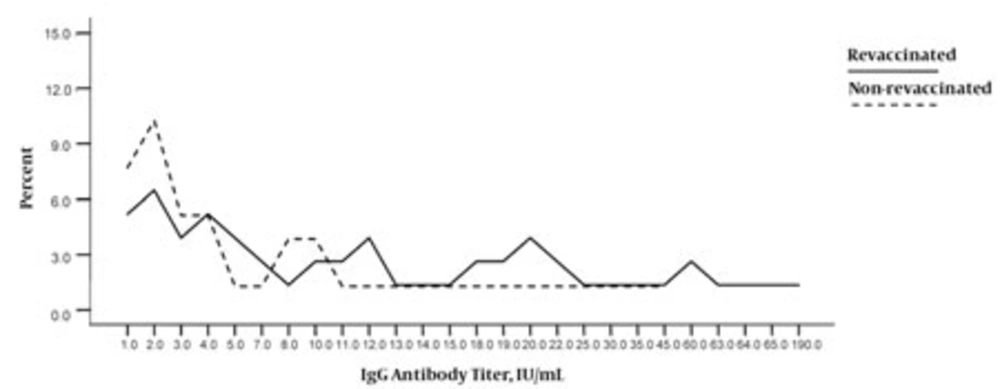
Table 2 summarizes the results of the IgG titer assessments for both groups according to this classification and Figure shows the distribution of individuals in both the groups on the basis of their serum IgG titers. IgM measurements were also classified into 3 groups,but the values obtained were not significantly different between the 2 study groups (data are not presented).
| Non-revaccinated, | 24 (72.7) | 7 (21.2) | 2 (6.1) | < 0.001 | 6.12 ± 8.8 | 33 (42.3) | < 0.001 |
| IU/mL | |||||||
| Revaccinated, IU/mL | 16 (35.6) | 8 (17.8) | 21 (46.7) | < 0.001 | 31.8 ± 21.2 | 45 (57.7) | < 0.001 |
| Total | 40 (51.3) | 15 (19.2) | 23 (29.5) | - | 78 (100.0) | - |
aChi square test
bIndependent samples t test
5. Discussion
The global coverage of measles vaccination increased from 15% in 1980 to around 80% in 1990, resulting in a 95% reduction in death and 90% reduction in cases, in comparison with the pre-vaccination era obtained in 1995. However, actual figures vary in different countries. The global measles vaccination coverage increased rapidly up to the year 1990 but remained static at around 80% for most of the 1990s mainly because of low or even sometimes falling coverage rates in many developing countries, particularly in sub-Saharan Africa and the Middle
East (2).
This feature, together with recurring localized outbreaks and epidemics of varying magnitude, promoted the health policy makers to plan new strategies such as mass vaccination campaigns. Immediately before the mass vaccination campaign in our country, measles antibody tests yielded negative results in 22.5% of Iraniansoldiers (3). In an Ethiopian study in 1,928 children, aged 9 month to 5 years, 45% tested positive for measles antibodybefore the vaccination campaign and 85% tested positive after the campaign (4). According to initial reports, the mass vaccination campaign in Iran has been successful because new outbreaks of measles have beeninfrequent (5). Vaccine production in Iran was started by the Razi Institute in 1970, and the immunization program was launched in 1976 by the Ministry of health and health research institutes in Iran. In 1996, mass vaccination for measles control was performed for 7 million children before this can be omitted the mass campaign implemented in 2003, and a second dose of mumps, measles, rubella (MMR) vaccine has been added to the routine vaccination program since 2004. To date, we do not have a routine screening program for vaccine preventable diseases for health care workers (HCWs) and students. One study in 1981 showed low immunity levels among children aged below 3 years, and another study in 2000-2001 reported low antibody titers in children aged below 18 months, especiallyinfants under 9 months of age (6, 7).
In 1997, a study conducted in Iran showed that out of 2,767 persons with eruptive disease with a clinical diagnosis of measles, 39% were confirmed to have measles according to a positive hemagglutination inhibition (HI) test while 61% had not been vaccinated against measles (8). The immunity rate, as tested by antibody levels, in our country was around 73.3% in 2003, and this is a risk factor for serious measles epidemics (9). On the basis of an unpublished report, the number of suspicious measles cases in Iran can be estimated to be 11,605, and there were 1,096 confirmed cases of the disease in 2002; these figures decreased to 765 suspicious cases and 14 confirmed cases together with a 99% decrease in mortality rate in 2005 (10).
There are different approaches for antibody determination, such as antibody neutralization, ELISA, rapid dot-immunobinding assay, and detection of measles virus (MV) RNA by nested real-time PCR (11, 12, 13). Detection of viral antigenic variants helps in epidemiologic evaluation of the virus’s entry into the community (14). Identification of primary and secondary measles vaccine failure (i.e., failure to seroconvert after vaccinationand waning immunity after seroconversion, respectively) by measuring IgG avidity is very important for evaluating the success of measles control programs in developing countries (15).
In the present study, only 46.7% of revaccinated individuals had protective levels of the IgG antimeasles antibody, which is far from the optimal target that is assumed ideal for protection status in vaccinated students can be more than 80%. Although our study sample is not representative of our population, these findings may suggest the need for nationwide investigation or at least facilitate some local studies about primary and secondary measles vaccine failure in the community. The post-vaccination levels of anti-measles antibody varies with different factors, e.g., a study conducted in West Africa showed that anti-measles antibody concentration 5 to 7 years after vaccination was higher when checked in the rainy season than that in the dry season. Perhaps malaria and other infections cause fluctuations in antibody responses to vaccines and accelerate the decay of these responses (16).
It is also known that subclinical infections can occur in patients with a previous history of vaccination because of the gradual decrease in the levels of specific antibodies to measles (secondary vaccine failure). A large number of children in the UK are born to mothers with a history of measles vaccination and therefore, are susceptible to measles; if these children are exposed to the disease from international travelers, they are at a risk for contracting measles. Children in the US may have similar risks (17, 18). In conclusion, continuous monitoring of anti-measles antibody by an expert team is recommended in order to eliminate measles in our country.