1. Background
Neuroblastoma is the most common extracranial solid tumor in children younger than 5 years (1) and it is a leading cause of death due to the development of malignancy in this population. Because of the extent of the disease at diagnosis, prognosis is generally poor in most patients (2). However, this malignancy can also exhibit favorable characteristics, including the possibility of evolving into a more benign form, or regressing spontaneously (2). Clinical evaluation of the disease, including in vivo imaging and bone marrow examination, and the age of the patient at diagnosis allows a definitive prognosis to be made in the majority of cases (3).
Progress in the management of neuroblastoma requires precise evaluation, and this is based on the characterization of a number of biochemical and molecular abnormalities (4). Molecular characterization of neuroblastoma could be a better tool in the diagnosis of tumor aggressiveness and progression. There are several molecular markers which have been variously related to patient survival; deletion of the short arm of chromosome 1 (5), DNA ploidy (6), and the expression of nerve growth factor receptor, encoded by the TRKA gene (7). However, in clinical practice, MYCN amplification has routinely been used to determine diagnosis as a basis molecular marker (2, 5). MYCN amplification seems to correlate with biological features such as, advanced disease stage and rapid tumor progression of neuroblastoma (8, 9).
Oncogene amplification is a common DNA alteration in neuroblastoma, causing an increase in encoded protein synthesis (10). Several methods have been suggested to determine oncogene amplification, based mainly on; Southern or dot blot methods (11, 12), quantitative polymerase chain reaction (PCR) (13, 14), and fluorescence in situ hybridization techniques (15). The PCR is a relatively rapid and simple method. PCR has many benefits over conventional methods for detecting gene amplification; it does not require radio-labeled probes and requires less DNA samples. Such diagnostic technique benefits are of special interest in small tumor samples which have been obtained by bone marrow aspiration or fine needle aspiration (16).
Shafa Medical, Educational and Research Center is a major referral hematology and oncology hospital in the southwest of Iran and many children with neuroblastoma have been managed in this center. To the best of our knowledge, no study has been performed to investigate the effect of N-myc amplification on median survival rates in children with neuroblastoma.
2. Objectives
Therefore, we conducted a study to determine N-myc amplification in children with neuroblastoma using standard polymerase chain reaction (PCR) and to show the impact of its amplification on patients’ median survival.
3. Patients and Methods
This study is an analytical historical cohort research. All of the children with a primary diagnosis of neuroblastoma admitted to the Shafa Hospital from April 1999 to April 2010, and where their paraffin embedded samples were also available, entered the study. In each case documentation was made of the following; demographic indicators, stage of tumors and whether the patient was alive or dead. Forty patients were included in this study. There were 22 (55%) males and 18 (45%) females. The age of the patients ranged from 6 months to 11 years; 12 patients ≤ 2.5 years of age and 28 were > 2.5 years. Patients staging were performed according to the International Neuroblastoma Staging System (INSS). Of the 40 patients, one (2.5%) was in stage II of the disease, 10 (25%) were in stage III, 27 (67.5%) were in stage IV and two (5%) were in stage IV-s (Table 1).
| 2 | 1 (2.5) |
| 3 | 10 (25) |
| 4 | 27 (67.5) |
| 4s | 2 (5) |
In the first step, paraffin-embedded tissue sections were cut and then the paraffin was dissolved in xylene. After precipitation of the sample and removal of the supernatant, the residual xylene was removed by washing with ethanol. In the next step, the DNA was extracted. For assurance about the quality of the extracted DNA, both purity and concentration were measured. “High Pure PCR template preparation kit; Roche Diagnostics Corporation” was selected for chemical/enzymatic analysis of cell lysis, and nuclease inactivation, while adsorption chromatography was used for nucleic acid purification. The kit contains 5 different buffers, proteinase K, high pure tubes with pre-packed silica filters and collection tubes. In brief, the starting material is lysed by incubation with lysis buffer and proteinase K to break open cell membranes and expose DNA and RNA. Binding buffer is added to inactivate the nucleases and the solution is transferred into filter tubes and centrifuged for 2 minutes. After three washes with different buffers, the nucleic acid bound to the glass fiber filter was purified and could be eluted with the elution buffer into a sterile 1.5 micro centrifuge tube. The Eppendorf BioPhotometer (Eppendorf, Hamburg, Germany) is the best instrument for quick and routine measurements of the optical density of samples at predefined wavelengths. With this device, we estimated the concentration of the extracted DNA. DNA concentration can be determined by measuring the absorbance at 260 nm (A260) in a spectrophotometer. The ratio of the readings at 260 nm and 280 nm (A260/ A280) provides an estimate of DNA purity with respect to contaminants that absorb UV light, such as proteins. The fragmentation of purified nucleic acid was checked using denaturing agarose gel electrophoresis. Quantitative PCR were performed in the Corbett Rotor-Gene 6000 (Corbett Life Science) in a 25 µL final volume. The PCR mixture contained 2.5 μL 10x buffer, 1.5 µmol MgCL2, 20 nmol/L of each primer, 10 nmol/L of each probe, 1 unit of Taq DNA and 100 ng of extracted DNA.
Both genes (NAGK gene and MYCN oncogene) were amplified for 120 seconds at 95° C in the first step, followed by 45 cycles of 30 seconds at 95° C, 30 seconds at 60° C, and 30 seconds at 72° C. The degree of amplification for each sample was derived from the ratio of the number of molecules of MYCN to the number of molecules of NAGK reference gene. Only samples in which the MYCN/NAGK ratio was three or more were considered as amplified for this oncogene. Statistical analysis was performed by the Kaplan-Meier method (22).
4. Results
N-myc amplification was detected in 32/40 (80%) of the patients. The N-myc/NAGK ratio of the 32 amplified samples varied from 3 to 2 200. Of these 32 patients with N-myc amplifications 9 (28.1%) were ≤ 2.5 years of age, 16 (50%) were 2.6 - 5 years of age and 7 (21.9%) were > 5 years of age (Table 2). Fifteen of these 32 (46.9%) patients were males, while 17/32 (53.1%) of the patients were female. Nine of the 32 (28.1%) were in stage III, 21 (65.6%) were in stage IV, the remaining 2 (6.2%) were stages II and IV-s tumors. Ninety percent of stage III and 77.8% of stage IV tumors included in the study had N-myc amplification. In children ≤ 2.5 years of age, the incidence of N-myc amplification was 30% in stage III tumors and 14.8% in stage IV tumors. In children > 2.5 years of age, the incidence of N-myc amplification was 60% in stage III tumors and 62.9% in stage IV tumors. As shown in Table 2, with increasing age and stage of the tumor N-myc amplification increased.
| 2 | 1 (8.33) | 0 | 0 | 0 |
| 3 | 3 (25) | 0 | 6 (21.4) | 1 (3.5) |
| 4 | 4 (33.3) | 2 (16.6) | 17 (60.7) | 4 (14.2) |
| 4s | 1 (8.33) | 1 (8.33) | 0 | 0 |
| in age group | 9 (75) | 3 (25) | 23 (82.1) | 5 (17.8) |
| Total | 9 (22.5) | 3 (7.5) | 23 (57.5) | 5 (12.5) |
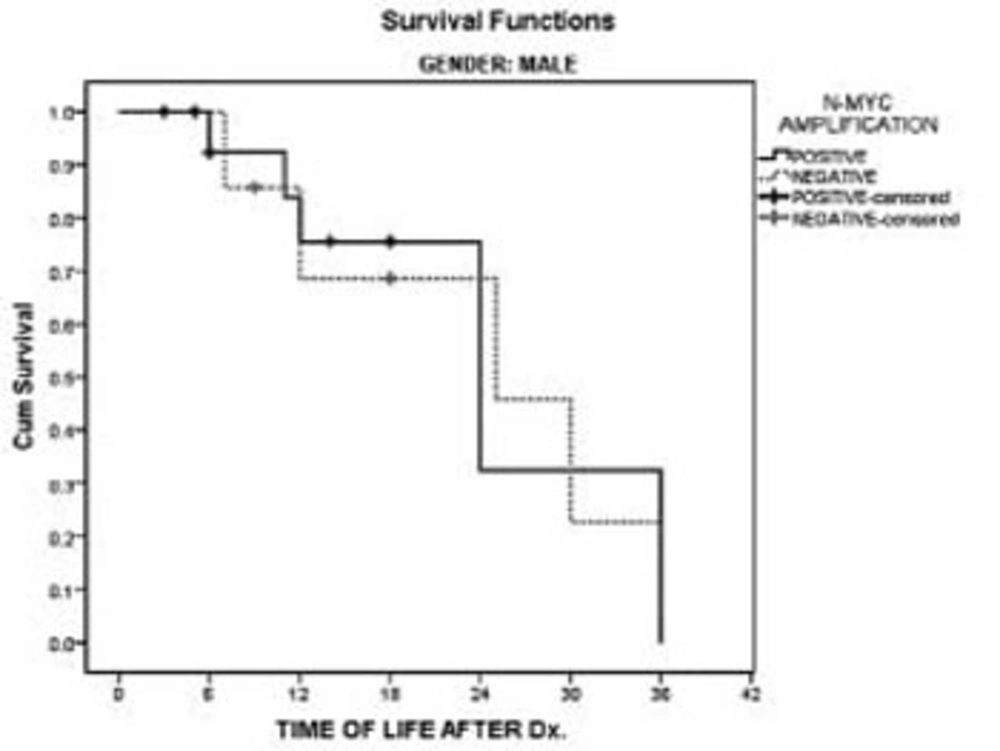
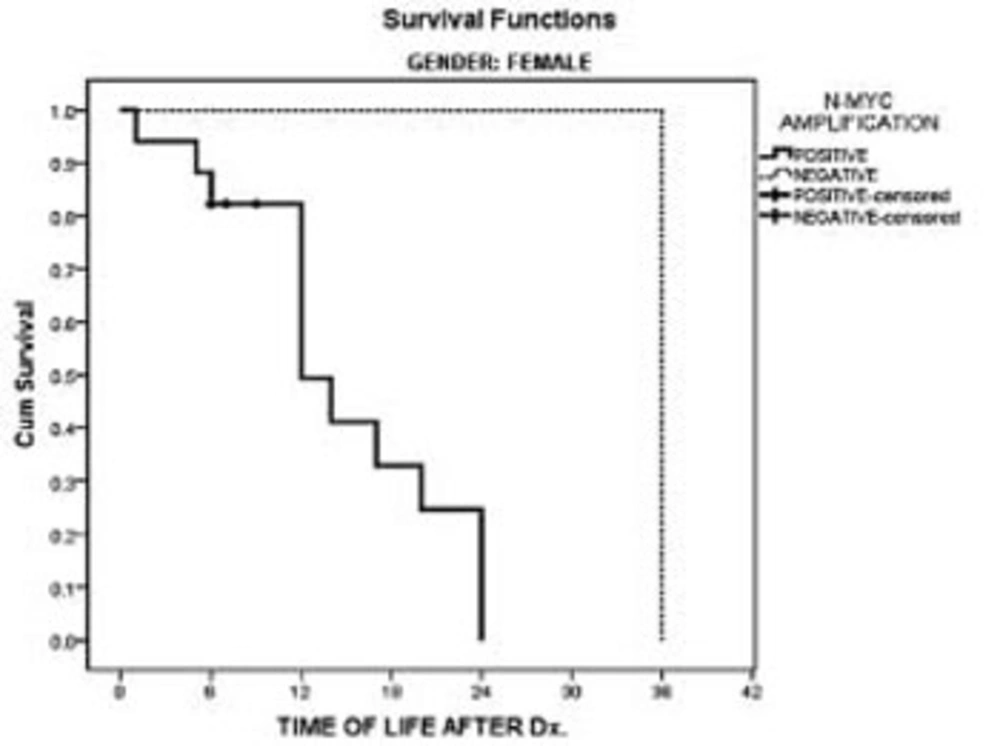
Patients’ charts showed that all of the children had died within 36 months after diagnosis. In the male’s survival rate, a comparison of patients with and without N-myc amplification had no statistically significant difference in this study. However, in the females, N-myc amplification had a significant negative impact on patients’ survival in comparison with females without N-myc amplification (P < 0.02). In females with N-myc amplification all of the patients had died within 24 months following diagnosis. (Figures 1 & 2). Overall patient median survival was longer in males either with or without N-myc amplification in comparison with females (P < 0.03).
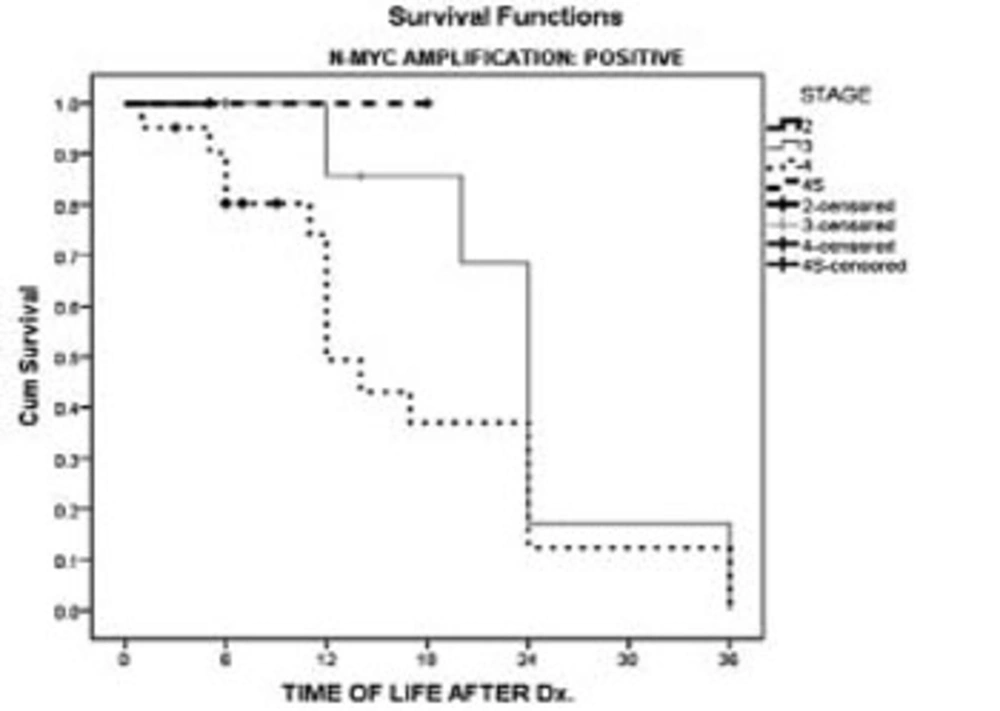
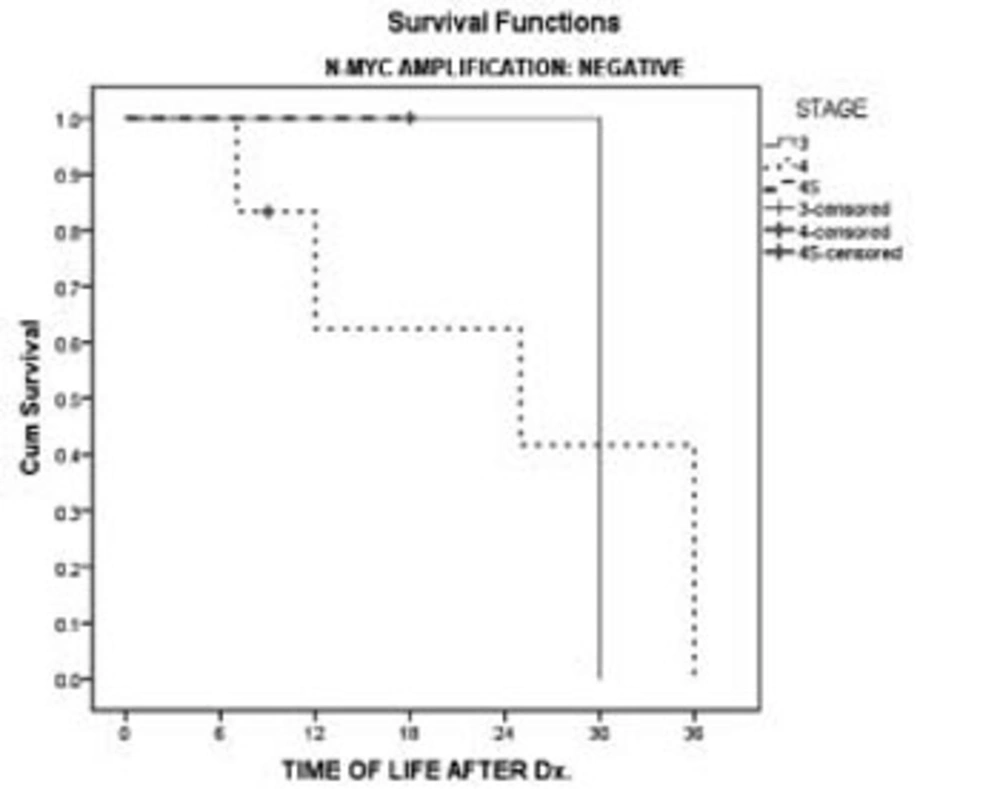
We have also taken into account the effect of N-myc amplification and stage of the malignancy on survival. Survival of the patients at stages 3 and 4 showed no difference between patients with or without N-myc amplification (Figures 3 & 4).
5. Discussion
The measurement of MYCN gene amplification in neuroblastoma is one of the most important clinical applications of oncogene amplification detection in oncology. Both prognosis and treatment of this tumor are deeply influenced by this molecular marker (2, 5, 17, 18, 19, 20, 21). Any improvement in the accuracy and reliability in the determination of MYCN amplification could represent an important tool in the management of this malignancy. Our data demonstrated that the measurement of MYCN amplification with a TaqMan reaction is easy, rapid, precise, and accurate. Our results confirmed the high incidence of MYCN amplification in neuroblastoma (2, 5, 17, 18, 19, 20, 21). We found oncogene amplification in 80% of the patients, with a major incidence in advanced tumor stages (2).
Previous studies have showed the effect of gender, stage, and N-myc amplification on median survival rates in patients with neuroblastoma. Our results showed that survival was no different in males with or without N-myc amplification, however, females without N-myc amplification had longer survivals compared with females who had N-myc amplification. Concerning the reasons for this finding we are not clear, but gender seems to be a more important prognostic factor than N-myc amplification in median survival rates of patients with neuroblastoma. Our results also suggest that N-myc amplification has a significant impact on median survival in females with neuroblastoma. Moreover, our results indicate that male children had greater resistance to the effects of N-myc amplification.
The results of our study are in accord with previous studies. In a study that was conducted in Italy, 49 children with neuroblastoma were investigated for N-myc amplification and were divided into three groups; group A (n = 23) included patients with no MYCN amplification, group B (n = 22) included patients with low amplification (between 2 and 8), and group C (n = 4) included patients with high amplification (≥ 9). The analysis of cumulative survival by Kaplan-Meier curves showed that patients with MYCN amplification had a significantly worse prognosis, compared with patients in whom the oncogene was not amplified (22).
Our results should be interpreted in the face of certain limitations. Our work was a retrospective cross-sectional study with only a small sample size, thus designing larger and longer term prospective studies on this subject are recommended. Also, we did not consider degrees of N-myc amplification on patient median survival and patients were only divided into two groups, with and without N-myc amplification.
In conclusion, the prognostic value of MYCN amplification in neuroblastoma is confirmed from our data. The analysis of cumulative survival curves confirmed that the prognosis is worse in patients with MYCN amplification. These data also validate the importance of correct measurement of oncogene amplification in the clinical evaluation of neuroblastomas to direct more aggressive therapies in patients with a higher risk of cancer progression. However, to obtain clear evidence of the possible clinical impact of a TaqMan assay for MYCN amplification measurement, larger groups of patients need to be studied.