1. Background
Sedation is defined as reducing patient’s awareness in order to diminish anxiety and physical stimulation sensation. Depth of sedation varies in a wide spectrum from mild sedation to complete anesthesia (1, 2). This can be achieved by administration of pharmacological or nonpharmacological methods, both of which could be considered for management and control of restlessness or anxiety in patients in the emergency room (ER) (2). Nonpharmacological methods include different approaches such as providing silent and relaxing environment, hypnotism, cognitive behavior therapy, etc. Pharmacological method consists of applying sedative, antiepileptic and analgesic agents (3). Naturally, there are different medications to induce sedation in pediatrics in the ER, including single drugs or combination of multiple medications. Nevertheless, there is still tendency to discover a novel medication for obtaining more appropriate and deeper sedation with fewer side effects (2). Early sedation in pediatric patients prevents them from experiencing psychological difficulties during physical examination or any procedure in the ER (2, 3). Medications could be administered via oral, intravenous (IV), intramuscular (IM) and subcutaneous (SC) routes or as aerosols; however, the easiest and most common route is oral (2).
Among the important roles of any emergency physician are optimizing the pediatric patients’ convenience, maximizing their comfort level, and reducing the side effects of sedatives as much as possible (2-4). There are very few studies on the administration of sedation in pediatrics by anesthesiologists before 1980 (3, 4). Charles and Theodor warned about the careless usage of sedation drugs, which could increase morbidity and mortality (4). That is why, the variety of medical associations such as anesthesiology, pediatrics, emergency medicine and pediatrics’ dentists in the United States, up to 12 societies, have their own recommendations for administration of sedatives (5-7).
2. Objectives
The present study was designed to assess the effectiveness of oral diphenhydramine-midazolam versus oral diphenhydramine administration for pediatric sedation in the ER.
3. Patients and Methods
After obtaining written informed consents from the parents of the children, we selected 100 infants, toddlers and children up to 13 years, referred to Imam Reza Hospital, Tabriz, Iran for suturing their wounds. The exclusion criteria were as follows: Refusal by parents, known allergy, underlying coexisting diseases such as convulsion, mental retardation, known psychological diseases, patients with adenoid hypertrophy, flu or common cold, deep injury with suspicious tendons cut or neurovascular damage, multiple trauma, decreased level of consciousness (GCS < 15), patients with lower-extremity ulcers, mucosal ulcers, wounds larger than 4 centimeters or with the depth of more than 0.5 centimeters. The sample volume was calculated using the following formula:
N = z² p (1 - p) ∕ d²
z = 1.96, P = 0.9
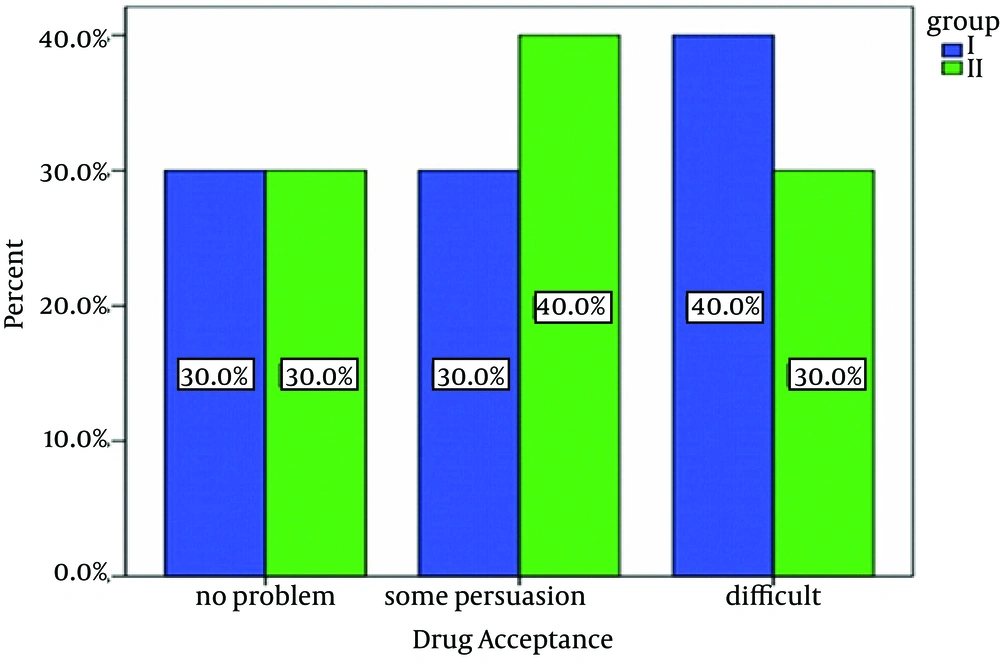
False estimation of 10% was suggested during the sedation induction. A sample volume of 35 patients was calculated which was considered 50 in this study. This study was double blind, incidental and prospective, and sampling was confidential and randomized. Patients were randomly divided to two equal subgroups of I and II, each of which containing 50 subjects by picking a ballot. Group I received 1.25 mg/kg diphenhydramine (Darou-Pakhsh 60 mL Syrup, Tehran, Iran) and group II received 1.25 mg/kg diphenhydramine plus 0.5 mg/kg midazolam (Aburaihan 5 mg/mL Amp, Tehran, Iran) orally. We add 20 cc of fruit juice to both groups' medications to improve their taste and administered them using a cup or syringe. The drug compliance and anxiety were categorized as follows:
1) No problem, 2) Some persuasion needed, 3) Difficult. After the drug administration, we asked the parents to lay down their children on the bed. Oxygen was applied either by nasal cannula or facial mask and the level of conscious and vital signs were monitored continuously. In the cases that needed local anesthesia, we selected those with the anxiety scores of more than one. Lidocaine 4% spray was applied before the injection of lidocaine. Lidocaine injection was repeated if the depth of sedation was scored less than four (45 minutes after starting oral sedation). Child behavior patterns such as crying, consciousness and movement were recorder by other staff based on the Houpt scale. An emergency medicine specialist prepared all medications in the ER; while, administration and filling out the forms were performed by a third specialist blinded to the types and dosages of medications. Pulse oximetry (Oxypleth Novametrix Medical System Inc. Wallingford, CT, USA) was used to monitor the patients' oxygenation. Two weeks after the discharge, we called the parents and followed any possible experience of complications such restlessness, insomnia, nightmare, enuresis, grouch, disobedience, or separation anxiety within this period. Statistical analysis was performed by SPSS version 17.01 (SPSS Inc., Chicago, Illinois) and relevant variants were assessed by chi-square and Mann-Whitney tests. P value < 0.05 was considered statistically significant. Flow diagram of the study is shown in Figure 1.
4. Results
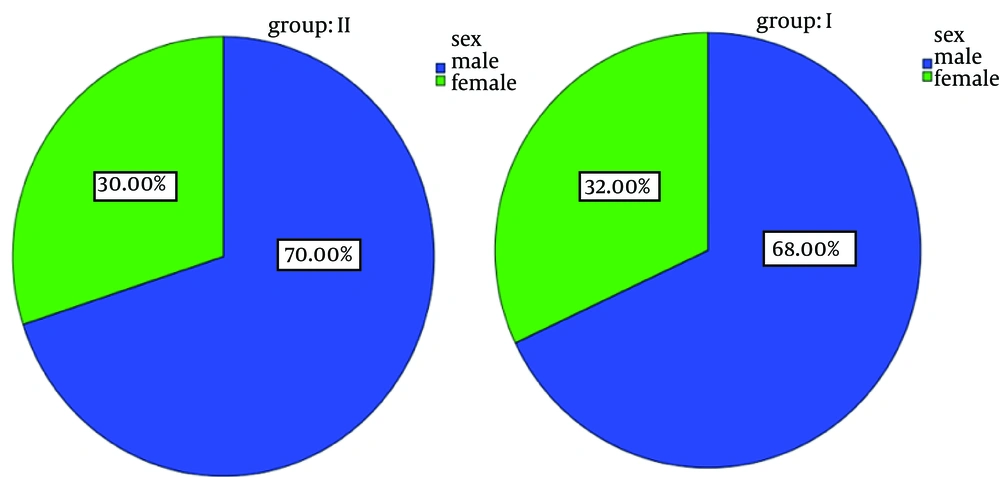
There was no significant difference regarding gender or age between both groups. Nearly 70% of all subjects in both groups were males and 30% were females (Figure 2). The average age was 4.9 years in group I and 5.5 years in-group II. There was no significant statistical difference regarding the drug compliance (P = 0.4) (Figure 3). In group I, none of the patients reached to even 2nd or 3rd levels of sedation depth within the first 5 minutes. Only 32.3% of group I members reached to the 2nd and 3rd levels of sedation after of 10 minutes. In group ІІ, 58% of patients in 5 minutes and 42% in 10 minutes reached to the 2nd and 3rd levels of sedation after of 10 minutes. The maximum time to achieve the optimal sedation was 16.13 ± 4.78 minutes in group I and 7.1 ± 2.49 minutes in group II (P < 0.0001). Behavior study of the children was obtained by phone calls to their parents 2 weeks after the discharge; restlessness and insomnia were less frequently seen in group II in comparison with group I (P < 0.0001) (Table 1).
| Behavior | Yes | No | P Value |
|---|---|---|---|
| Restlessness | < 0.0001 | ||
| Group I | 30 (60) | 20 (40) | |
| Group II | 10 (20) | 40 (80) | |
| Insomnia | < 0.0001 | ||
| Group I | 20 (40) | 30 (60) | |
| Group II | 4 (8) | 46 (92) | |
| Nightmare | NA | ||
| Group I | 0 (0) | 50 (100) | |
| Group II | 0 (0) | 50 (100) | |
| Enuresis | NA | ||
| Group I | 0 (0) | 50 (100) | |
| Group II | 1 (2) | 49 (98) | |
| Immoral | NA | ||
| Group I | 0 (0) | 50 (100) | |
| Group II | 0 (0) | 50 (100) | |
| Anorexia | 0.47 | ||
| Group I | 13 (26) | 37 (74) | |
| Group II | 10 (20) | 40 (80) | |
| Impatience | NA | ||
| Group I | 2 (4) | 48 (96) | |
| Group II | 0 (0) | 50 (100) | |
| Separation Anxiety | NA | ||
| Group I | 0 (0) | 50 (100) | |
| Group II | 0 (0) | 50 (100) |
a Abbreviation: NA, not available.
b Data are presented as No. (%).
c Group I received 1.25 mg/kg diphenhydramine; group II received 1.25 mg/kg diphenhydramine plus 0.5 mg/kg midazolam.
5. Discussion
Experiencing anxiety and fear prior to any medical procedure, particularly in children, could entirely affect the procedure. Any probable further damage could be decreased by reducing the stress and anxiety (8, 9). It has been well-studied that tranquilizers and sedatives are also of analgesic effects to some extent. However, to choose the most appropriate option, not only the sedation efficiency but also side effects or contraindications of the drugs should be considered. Most of the previously-performed studies focused on either single drug or multiple drug combinations such as ketamine, midazolam, atropine, diphenhydramine, glycopyrrolate, meperidin and fentanyl; in these studies, the administration route was either oral or IV (10).
Diphenhydramine, prescribed as a hypnotic-sedative agent, also has antihistaminic and anticholinergic effects. Paul and Jason compared the efficiency of oral midazolam and ketamine for inducing sedation in children before suturing their wounds; it was shown that ketamine was superior to midazolam and children who received ketamine tolerated the local anesthesia before the procedure much better than the ones received midazolam (7). In a similar study, Cengiz et al. compared the sedative effects of diphenhydramine-midazolam with single midazolam before MRI in children. In this study, deeper sedation onset was obtained much earlier in combination induction. In contrast, the children receiving single midazolam had lower and inadequate sedation levels. Nonetheless, in contrast with our study, there was no significant difference for sedation onset in both groups. Similar to our study, Cengiz et al. showed that diphenhydramine-midazolam had much more sedative properties in comparison with single midazolam in children before MRI, and it seemed that the combination medication had fewer side effects as well (11). In a study performed by Warner et al. combination of strawberry-flavored midazolam and ketamine, 20 minutes before operation in children aged 1 - 5 years, significantly reduced their stress and anxiety before general anesthesia (12). Munro et al. also showed that combination of midazolam and ketamine had noticeable impact on sedation of children before procedures with no significant side effect (13). In addition, in a study performed on maxillofacial surgeries, it was suggested that administration of ketamine, midazolam or glycopyrrolate before minor facial surgeries was associated with considerable effects in children; in addition, dysphoria and muscle rigidity, induced by ketamine, could be controlled by midazolam administration (14). In a study by Weber et al. it was revealed that intranasal administration of ketamine-midazolam prepared relaxed condition for the preschool children before general anesthesia (15). Most studies have highlighted the fact that combination medication has been more efficient in sedation of children compared to a single agent. Although some studies such as Younge et al. (7) or Taghiporanvari et al. (9) showed that sedation induced by midazolam as a single agent could be less efficient compared to other agents, other studies have suggested that combination of midazolam with other sedatives could play a central role in inducing sedation in children.
Our result also showed that diphenhydramine-midazolam is more significantly efficient in comparison with single diphenhydramine in reducing pain. Eskandarian et al. assessed the effect of midazolam-ketamine on behavior changes compared with midazolam-hydroxyzine for dental procedures (2). Similar to our study, ketamine-midazolam could control the movement in children successfully. Ketamine-midazolam, in comparison with hydroxyzine-midazolam, more efficiently induced hypnosis, controlled crying and body movement, and assessed the child's behavior. Our study showed that diphenhydramine-midazolam was more advantageous over single diphenhydramine in inducing sedation in children.
Overall, it could be concluded that diphenhydramine-midazolam could act as an appropriate medication to induce sedation in children in ER prior to numerous procedures. Combination of diphenhydramine-midazolam in comparison with single diphenhydramine provided higher quality of sedation, with fewer complications before diagnostic and therapeutic procedures in children.