1. Introduction
Tumors of the heart are rare, approximately three quarters of them are benign, and nearly half of the benign tumors are myxomas. About 75% of myxomas develop in the left atrium. Right atrial myxomas are infrequent and are observed in 15% to 20% of the cases (1). According to their location, the clinical manifestations of these tumors differ and some patients remain completely asymptomatic. Symptoms are essentially caused by pulmonary embolism, cardiac obstruction, and heart rhythm disorder (2).
2. Case Presentation
A four-year-old girl was referred to our department for a persistent cough. She had a previous history of exertional dyspnea. At admission to hospital, an apical systolic murmur was heard, the blood pressure was of 100/55 mm Hg, and the heart rate was regular at 105 beats per minute. The child did not show any sign of heart failure and the rest of the physical examination findings were unremarkable. Routine laboratory tests showed a biologic inflammatory syndrome with a high white blood cell count and a C-reactive protein level of 121 mg/L (1152.40 nmol/dL).
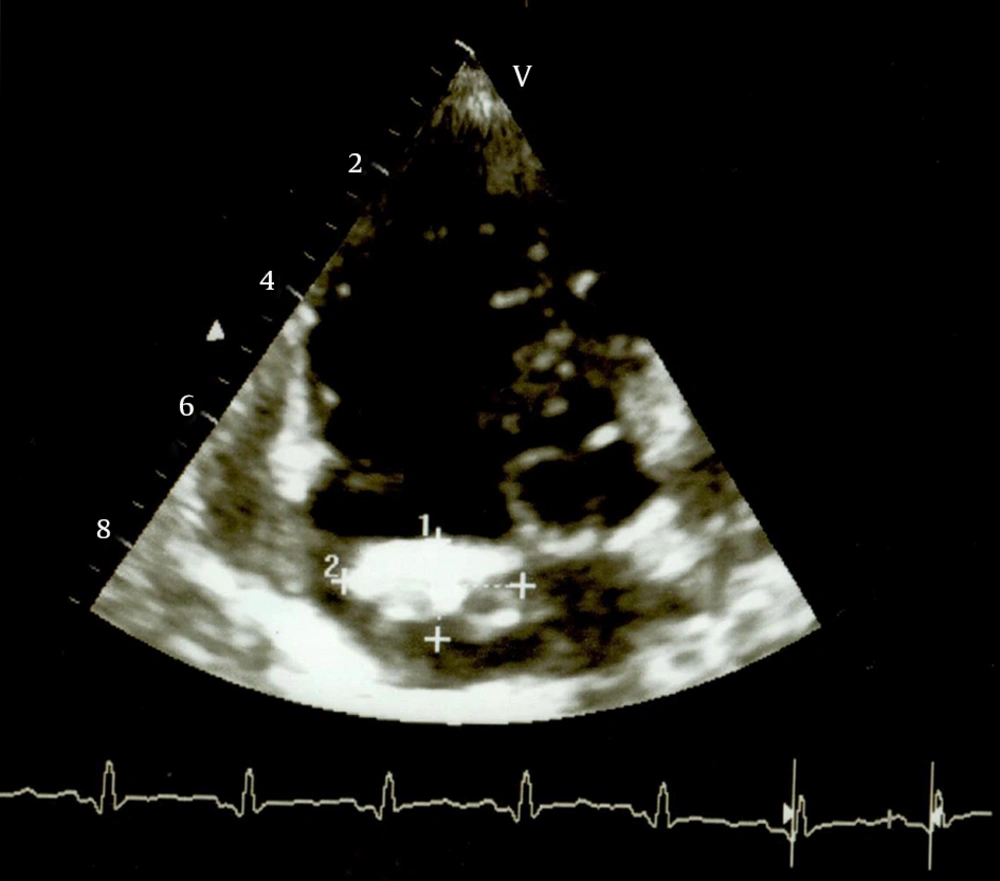
The electrocardiogram demonstrated a normal sinus rhythm and signs of right ventricular hypertrophy. Chest radiograph showed cardiomegaly with a cardiothoracic ratio of 0.64 with an enlarged right heart border and calcifications at the level of the right atrium. Pneumonia of the right lung base was also noted. Transthoracic echocardiography revealed an echogenic right atrial mass, of huge dimensions (3 cm2 in circumference), pedunculated and arising from the lower portion of the interatrial septum. This mass did not prolapse through the tricuspid valve into the right ventricle. The right atrium was dilated. The left cavities were of normal appearance and function. Pulmonary hypertension was noted. There was no pericardial effusion (Figure 1). These findings were suggestive of cardiac tumor and the antibiotics for treating patient's pneumonia were started. The preoperative assessments of the lesions were as follows:
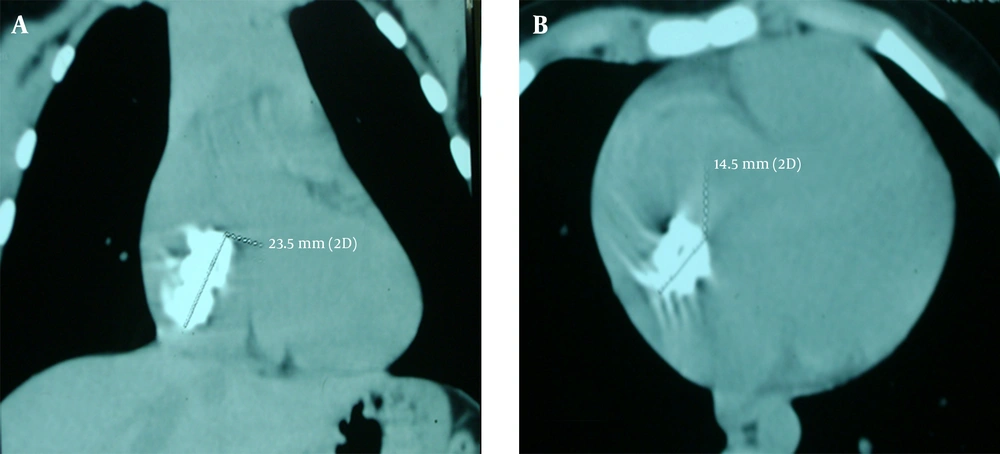
Computed tomography of the chest (Figure 2) showed a right atrial enlargement caused by a massive (24 × 14 × 13 mm) well circumscribed mass of homogenous density within the right atrium. Cardiac magnetic resonance imaging confirmed the tomographic findings. It revealed a right atrial pedunculated mass arising from the interatrial septum with a low T1 and high T2 signals. Holter rhythm monitoring showed neither cardiac arrhythmias nor conduction disturbances. The evolution was marked by the occurrence of syncope at rest. The holter rhythm was controlled and was still normal. This syncope was found to be related to an intermittent prolapse of the tumor through the tricuspid valve.
Our patient underwent median sternotomy under general anesthesia. We exposed the heart through a median sternotomy. The ascending aorta and both venae cavae were cannulated. The right atrium was opened with an oblique incision. The right atriotomy allowed showing a lobulated, calcified and non-friable tumor measuring 2.5 × 1.5 cm with a perforation of the upper side measuring 0.5 × 0.25 cm. This tumor had two bases of implantation on the atrial septum, one just above the foramen ovale and the other 1 cm above the inferior vena cava. The foramen ovale was permeable. No other visible defects were noted. We proceed to an excision of the entire tumor and its bases with a safety margin. Another small septal nodule of 3-mm diameter away from superior septal implantation was also removed. Reconstruction of the atrial septum with autologous pericardium was performed. The histopathologic examination confirmed the diagnosis of myxoma by showing calcified benign tumor proliferation made of a myxoid hypocellular tissue without atypia or mitosis associated with calcifications. In the follow-up after three years of surgery, the patient showed significant improvement with no evidence of recurring tumor.
3. Discussion
Cardiac myxoma is rare but represents the most common cardiac tumor. Its frequency ranges from 0.5% to 1% of the soft tissue tumors (2). The right atrial location is rare. Myxoma is localized, in order of frequency, in left atrium (75%), right atrium (20%), and ventricles (5%) (3). This is usually a single tumor and multiple forms occur most often in familial cases and in Carney's syndrome (2, 4). Right atrial myxoma is rare in pediatric population. Mallick et al. reported a similar case in a ten-year-old boy (5). Our patient was four and significantly younger despite no features of familial syndromes as Carney’s complex.
The clinical manifestations of right atrial myxoma are polymorphic and nonspecific. The clinical presentation depends on the location, number, size, and mobility of the myxoma (6). The usual mode of revelation is a heart failure in 70% of cases, followed by pulmonary embolism. There is also a risk of tumor prolapse through the tricuspid valve, which might lead to syncope. Cases of cardiac tamponade and sudden death in completely asymptomatic patients have been reported (6).
Similar to the case reported by Mallick et al. (5), our patient presented with exertional dyspnea as the predominant symptom. The evolution was marked by the occurrence of an uncommon complication; the girl presented syncope secondary to intermittent prolapse of the myxoma through the tricuspid valve. The chest radiograph is usually normal. It can sometimes reveal a dilated right atrium or calcifications (7) as seen in our patient.
The transthoracic echocardiography remains the most important and widely available method for the diagnosis of myxoma. This exam can provide information on the location, size, shape, and mobility of the mass. The tumor is usually round, movable, and generally pendent from the interatrial septum by a pedicle. Sometimes heterogeneous aspects of tumor can be found, which are due to calcified or bleeding areas (7). The myxoma diagnosed in our young patient was particular by its location, his important dimensions, and its prolapse through the tricuspid valve. The pulmonary hypertension, revealed by this exam, explains the exertional dyspnea in our patient.
Computed tomography of the chest can reveal a lobulated and heterogeneous mass with intratumoral calcifications. It can also determine the relationship of mass with the tricuspid valve and the interatrial septum (7, 8). Magnetic resonance appearances of the cardiac myxoma are heterogeneous. They are typically spherical or ovoid masses that might be sessile or pedunculated with low or intermediate T1 and high T2 signals (8).
Computed tomography of the chest and MRI were performed in our patient. They showed a typical aspect and specified the relationship of the tumor with the tricuspid valve and the interatrial septum. histopathologic examination remains the only way to confirm the diagnosis (3). The treatment of choice for myxoma is surgical removal (4). Complete resection of the tumor and its implantation base with a good safety margin is essential to cure the disease and preventing recurrence and subsequent reoperations (9). Our patient underwent an excision of the entire tumor and its bases of implantation with a safety margin to avoid recurrence.
Immediate postoperative mortality ranges from 0.1% to 3.6% (4). Arrhythmia is a common postoperative complication, which might require long-term medication. Recurrence develops in 2% of the patients and the rate is higher in familial cardiac myxomas (10). The recurrence can be explained by inadequate resection, intra operative implantation or embolization, totipotent multicentricity, inheritance, and metastatic recurrence (11). Our patient has been followed over a three-year period with a significant clinical improvement and no signs of recurrence of the myxoma.
Cardiac myxomas are often detected incidentally in asymptomatic individuals; however, atrial myxomas might be a rare cause of syncope, especially in young patients. Echocardiography is essential to confirm the diagnosis and urgent surgical resection is the treatment of choice. The risk of recurrence and metastases justify regular echocardiographic monitoring.