1. Background
Urinary tract infection (UTI) is of great clinical importance owing to considerably high morbidity and mortality rates among children. Effective antimicrobial therapy for UTI is important and can reduce adverse events in this age group. Most recent studies, particularly in developing countries, on the antimicrobial susceptibility of bacterial pathogens causing UTI in children have shown high levels of antibiotic resistance in clinical settings (1-4). Some population-based surveys in North and Latin America have shown that resistance to commonly used antibiotics, such as trimethoprim-sulfamethoxazole, has doubled in uropathogens (5). Similar studies on young Iranian children with UTI have also revealed markedly high resistance to commonly used antibiotics, such as vancomycin, cloxacillin, amoxicillin, and some cephalosporin subgroups (6). In addition, it was suggested that the prevalence of antimicrobial resistance in young children with UTI is dramatically increasing and varies among different geographical and regional locations and various socioeconomic statuses (7). Increased antibiotic resistance is related to a variety of risk factors, including prior antibiotic exposure, urinary malformations, and the use of prophylactic antibiotics (8-10).
Owing to the slightly higher prevalence of UTI in our population compared to other developing countries (11) and the higher rate of congenital anomalies predisposing Iranian children to UTI (12), it is necessary to identify the bacterial agents responsible for UTI and to determine their sensitivity to antibiotics in our child population.
2. Objectives
The present study was designed to determine the common bacterial pathogens causing UTI and their antimicrobial resistance patterns in Iranian children.
3. Patients and Methods
This research is a cross sectional study on 114 children with culture-proven UTI treated in the pediatric ward of Najmieh hospital in Tehran over a 3-year period. The sampling strategy was convenient method. We didn’t check normal assumption. The study plan was approved by the scientific and ethic committee of Baqiyatallah University of Medical Sciences and there are no conflict in treatment of patients during the study. The subjects were classified into 3 age groups: neonates (< 28 days, n = 45), infants (28 days to 2 years, n = 41), and children (> 2 years, n = 28). Cultures were obtained from midstream-collected urine by using the suprapubic method in infants and small children and by the midstream method in toilet-trained children. Antibiotic sensitivity was tested using the disc diffusion technique (Kirby-Bauer’s technique) by using various antibiotic discs according to National Committee for Clinical Laboratory Standards (13). Susceptibility to the following drugs was tested: amikacin, ceftriaxone, ceftizoxime, trimethoprim-sulfamethoxazole, cefotaxime, imipenem, cephalexin, gentamicin, cefalotine, chloramphenicol, nalidixic acid, cefixime, ceftazidime, and nitrofurantoin. A urine culture was considered positive when it resulted in one or more colony from a urine specimen collected by the suprapubic method and greater than or equal to 100,000 colonies by the midstream method in children with febrile disease or urinary symptoms. For statistical analysis, organisms with intermediate susceptibility were considered resistant. A renal ultrasonogram was also obtained for all studied patients to identify or rule out urinary malformations.
The quantitative data’s distribution was checked by K-S and the differences and association was analyzed by using Chi2 and exact fisher test for qualitative data and Steudent T-test and Mann Whitney-U for quantitative data. All statistical analyses were performed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). P ≤ 0.05 was considered statistically significant.
4. Results
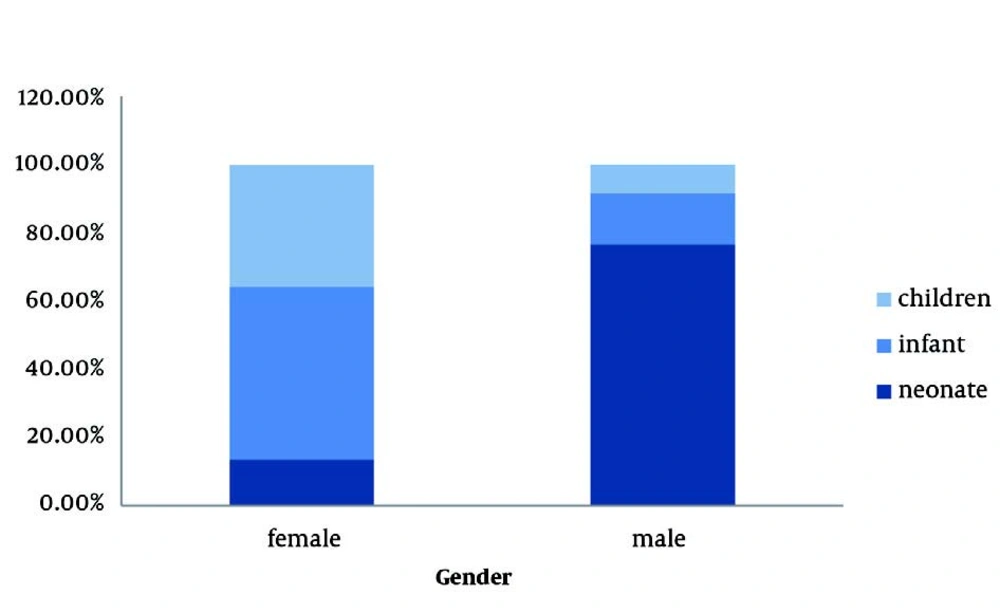
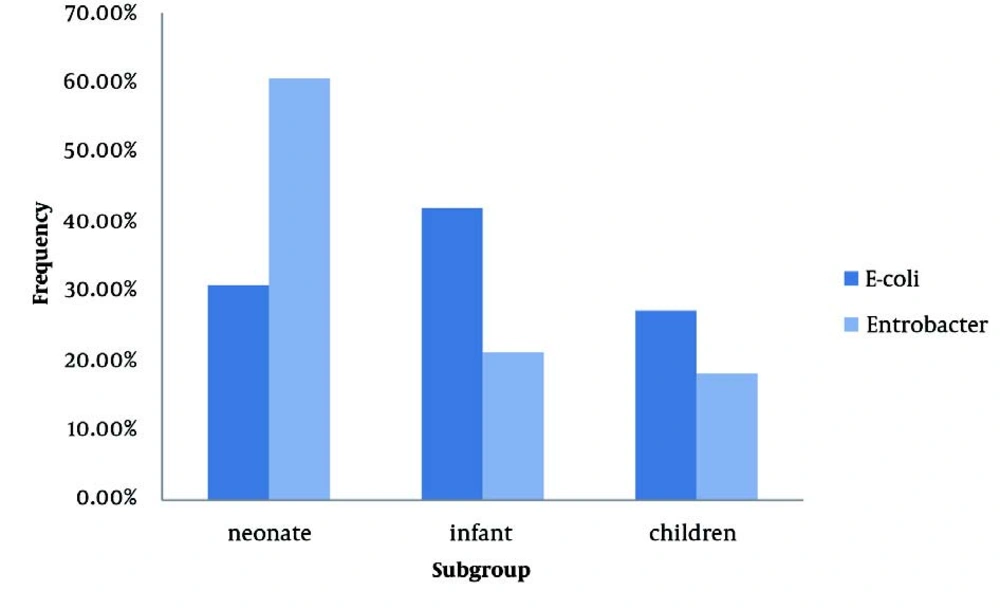
Among the studied infants and children, 40 (58.8%) were girls and 29 (41.2%) were boys. Boys were significantly younger than girls, and so, 34 (76.6% ) of boys and only 11 (23.4%) of girls were categorized in the neonate group (P < 0.001, OR: 21.09, 95% CI: 7.96-55.86) (Figure 1). Patients had a mean erythrocyte sedimentation rate (ESR) of 38.96 ± 3.95 mm/h and a mean white blood cell (WBC) count of 12,403.57 ± 5,233.60 μmol/L. The most frequently cultured pathogens included Escherichia coli 81 (71.7%) and Enterobacter 33 (28.9%). However, the frequency of these pathogens significantly differed across the 3 age groups. Specifically, the incidence of E. coli was higher in infants and children, and Enterobacter was more frequent in neonates than in the other groups (Figure 2). There were no significant differences between boys and girls in the frequency of cultured E. coli (61.7% vs. 77.6%) or Enterobacter (38.3% versus 22.4%). The antimicrobial susceptibility of the studied isolates is shown in Table 1. Imipenem was the most active agent against E. coli isolates (susceptibility, 97.3%), followed by ciprofloxacin (90.4%) and amikacin (82.9%). Trimethoprim-sulfamethoxazole, cefalotine, and cephalexin were the least effective agents, with 76.3%, 75.0%, and 73.7% of E. coli isolates exhibiting resistance, respectively. Imipenem and ceftizoxime were the most efficient antimicrobial agents against Enterobacter, with sensitivity rates of 85.2% and 71.4%, respectively. Nitrofurantoin, ceftazidime, and cefalotine were the least active agents against Enterobacter, with resistance rates of 92.3%, 66.7%, and 62.5%, respectively. Compared to E. coli, Enterobacter had a higher resistance rate to ceftazidime, amikacin, imipenem, gentamicin, and nitrofurantoin (Table 1). However, E. coli had higher resistance rates to cephalexin and trimethoprim-sulfamethoxazole than Enterobacter. Ninety-six patients (84.2%) had normal renal-bladder ultrasonograms. In the other cases, bladder ultrasonograms showed abnormalities, including bladder wall thickness, bladder wall irregularity, and residual urine. The frequencies of these abnormalities were comparable in boys and girls (12.8% vs. 17.9%, P = 0.458).
| Cephalosporin No. (%) | |||
| Cefotaxime | 19 (24.1) | 30 (29.4) | 0.557 |
| Ceftriaxone | 20 (25.0) | 14 (41.4) | 0.082 |
| Ceftizoxime | 18 (22.6) | 9 (28.6) | 0.458 |
| Cephalexin | 59 (73.7) | 11(33.3) | < 0.001 |
| Cefalotine | 61 (75.0) | 20(62.5) | 0.181 |
| Cefixime | 22 (27.9) | 14(42.9) | 0.120 |
| Ceftazidime | 21 (25.6) | 22 (66.7) | < 0.001 |
| Others, No. (%) | |||
| Amikacin | 13 (17.1) | 14 (41.9) | 0.005 |
| Trimethoprim-sulfamethoxazole | 62 (76.3) | 18 (55.2) | 0.026 |
| Imipenem | 2 (2.7) | 5 (14.8) | 0.015 |
| Gentamicin | 19 (23.4) | 17 (53.3) | 0.002 |
| Chloramphenicol | 28 (35.0) | 16 (50.0) | 0.137 |
| Nalidixic acid | 31 (38.4) | 11 (33.3) | 0.609 |
| Nitrofurantoin | 30 (37.2) | 30 (92.3) | < 0.001 |
Resistance to the Antimicrobial Agents Among Escherichia Coli and Enterobacter Isolated From Children With Urinary Tract Infections
5. Discussion
In the present study, the most frequently detected pathogen among children with UTI was E. coli (71.7%), followed by Enterobacter (28.9%). The frequency of E. coli infection is similar to that in previous reports from different regions of Iran and other developing countries. E. coli is the leading uropathogen and was isolated from 56.6–84.6% of Iranian children with febrile UTI (6, 11, 14, 15); this isolation rate had a wider spectrum in other countries, ranging from 37.3% (2) to 87.0% (4). Furthermore, the frequency of Enterobacter isolation in this study differed considerably from that of other similar surveys that isolated Enterobacter from urine cultures in only 2.3% to 3.7% of Iranian children with UTI (6, 14). Previous studies identified Enterobacteriaceae as the predominant pathogen in children with UTI (16-18). Similar to the findings of other studies, we more commonly isolated Enterobacter in neonates with UTI (44.4%) than in infants (17.1%) and children (21.4%). This higher incidence of Enterobacter infection may be due to hospitalization of neonates, especially in neonatal intensive care units (19).
Although boys in this study were significantly younger than girls, the frequencies of positive urine cultures were similar between the 2 groups. Farajnia et al. reported that the rate of positive cultures was nearly 2 times higher in females than in males (15). The male to female ratio in a report by Sharifian et al. was 1:2 (14). In other studies, UTI occurred in 1.5 to 5 times as many males as females during the neonatal period (20-22). Gender-specific differences in the rates of UTI may depend on the age. Boys are more commonly infected during the first 3 months of life, and after the first year, symptomatic UTI is more frequent among girls. Similarly, asymptomatic bacteriuria is more frequently detected in boys than in girls during the first 12 months of life. Thereafter, the incidence decreases markedly in boys but increases in girls (23).
In the present study, we showed that among the tested antibiotics, imipenem, ciprofloxacin, and amikacin were the most active agents, whereas trimethoprim-sulfamethoxazole and cephalosporins, such as cefalotine and cephalexin, were the least active agents against E. coli isolates. The resistance rates of E. coli in this study were also different from those reported in similar studies among both our population and in other nations. Modarres et al. found that E. coli was most sensitive to aminoglycosides and had lowest resistance to nalidixic acid (6). In a study by Sharifian et al., E. coli was shown to be most sensitive to ceftriaxone, ceftizoxime, and cefotaxime (14). Similar to our study, Movahedian found that E. coli isolates were most susceptible to amikacin and were most resistant to cephalexin (24). In addition, another study showed the highest susceptibility to amikacin (97.8%) and resistance to cephalexin was reported in 24% of cases (25). Other studies showed that the resistance of E. coli to first generation cephalosporins has increased by up to 20% in recent years (26). Based on our findings, imipenem, ciprofloxacin, and aminoglycosides, such as amikacin, are appropriate for initial empirical intravenous therapy for UTI among Iranian children, despite an increased resistance rate of E. coli isolates. Aminoglycosides are considered safe for the treatment of UTI in children and minimally impact the intestinal flora; therefore, there is a low rate of resistance against them (17). The efficacy of these drugs has been shown particularly in those who are receiving prophylactic antibiotics because of a high resistance rate to third-generation cephalosporins (8).
Our study found that nitrofurantoin had a moderate antibacterial effect against E. coli, with a resistance rate of approximately 60.0%. However, in previous studies, nitrofurantoin was found to be very active against E. coli isolates, with a susceptibility rate of 96.3% to 97.8% (3, 4, 7). Some studies even recommended nitrofurantoin as the first choice among oral antibiotics for prophylaxis and treatment of UTI in children (27).
In the present study, imipenem was the most effective antimicrobial agent against Enterobacter, but nitrofurantoin and almost all of the cephalosporins were the least active agents against Enterobacter. Although some recent studies have shown that most Enterobacteriaceae strains were susceptible to third-generation cephalosporins (28), others found that 31% of Enterobacter spp. infections in intensive care units were not susceptible to third-generation cephalosporins (29). In addition, a high rate of Enterobacter resistance to fluoroquinolone, beta-lactam, and cephalosporin antibiotics has been reported in European countries, South America, Asia, and Pacific regions owing to the production of extended-spectrum beta-lactamases (30). Therefore, because of its efficacy against antibiotic-resistant Enterobacter, imipenem is a suitable alternative therapeutic for the treatment of UTI caused by this pathogen in children.
In summary, the low susceptibility of different UTI-related isolates to nitrofurantoin and other common antibiotics, particularly broad-spectrum agents, is mainly the result of broad or inappropriate use of potent antibiotics in hospitals and changing the treatment approach without consulting a physician. Therefore, reducing the prescription of a particular antibiotic and informing patients about appropriate drug use can decrease the resistance to various antibacterial agents of common uropathogens causing UTI in children.