1. Introduction
Hyperoxaluria is a serious metabolic disorder. The autosomal recessive inherited Primary Hyperoxaluria (PH) types I, II, and III are caused by defects in glyoxylate metabolism due to specific hepatic enzyme deficiencies. Kidney damage is caused by deposition of oxalate crystals that induce renal epithelial cell injury, and inflammation is present in all forms, followed by reduced oxalate elimination and consequent systemic deposition of calcium oxalate crystals (1, 2).
Secondary hyperoxaluria mostly occurs in digestive diseases leading to increased intestinal absorption (2-6). Other rare causes include chronic pancreatitis (7), ethylene glycol (8), vitamin C (9), ingestion of foods with high levels of oxalate (10-12), and complication of some medications (3, 13, 14).
The current report present a case of idiopathic calcium oxalate nephropathy due to its rarity. Awareness of PH and measurement of plasma and urinary oxalate are essential, because early diagnosis, molecular sub typing, and prompt initiation of treatment are of vital importance, resulting in significantly improved outcome (2, 15).
2. Case Presentation
A 4-month-old male was referred to Mofid Children’s hospital due to high serum creatinine level, detected during his admission to another hospital for bronchiolitis. There was no history of urinary symptoms or pain. Laboratory test results were as follows, sodium 120 mEq/L (normal range: 135 to 148), potassium 4.3 mEq/L (normal range: 3.7 to 5.6), blood urea nitrogen 14 mg/dL (normal range: 5 to 17), creatinine 2.6 mg/dL (normal range: 0.8 to 1.3), calcium 8 mg/dL (normal range: 9 to 11), phosphorus 4.6 mg/dL (normal range: 4 to 7), uric acid 6.9 mg/dL (normal range: 2 to 5), and lactate dehydrogenase 522 IU/mL (normal range: up to 1100). C3, C4, CH50, ANA and anti ds DNA were within normal limits. Complete blood count (CBC) showed hemoglobin 6 g/dL (normal range: 9.5 to 13.5), hematocrit 18.6% (normal range: 29 to 41); mean corpuscular volume (MCV): 67.4 fL (normal range: 74 to 108), Mean Corpuscular Hemoglobin (MCH) 21.7 pg/cell (normal range: 25 to 35), MCHC 32.7 g/dL (normal range: 30 to 36), and platelet 226 × 103/µL (normal range: 150 to 400 × 103). Urinalysis revealed specific gravity: 1.010, PH: 5, white blood count (WBC): 12 to 16/HPF, red blood count (RBC): 0 to 1/HPF, and protein: negative.
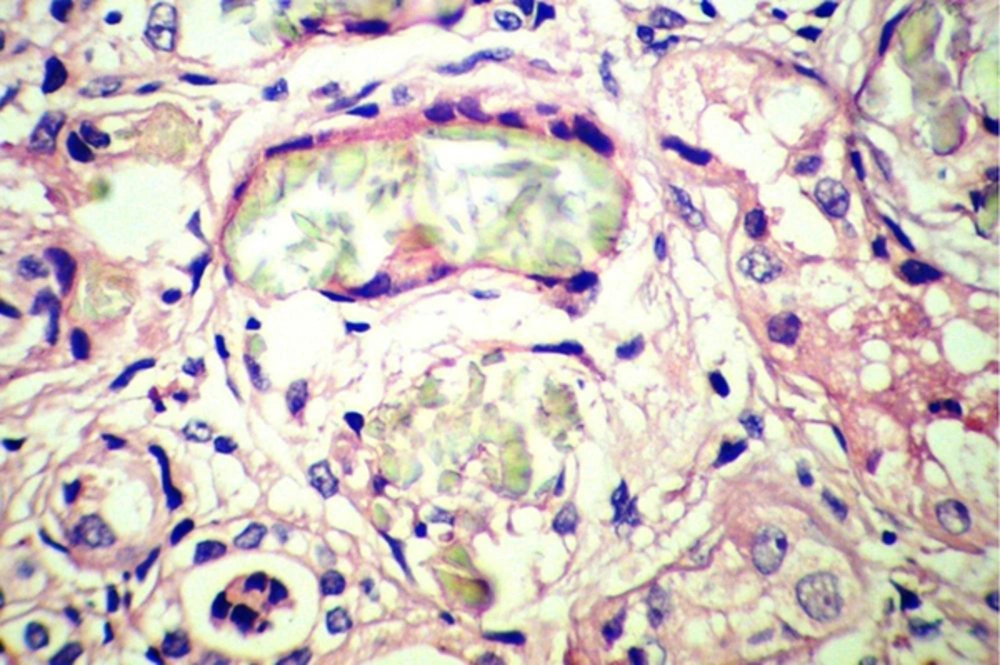
Sonography showed increased echo pattern and differentiation of both kidneys, associated with calcification of medullary papilla (nephrocalcinosis). The patient underwent bilateral open kidney biopsy, which revealed many intra-tubular and interstitial polarizing crystals, resembling calcium oxalate crystals (Figure 1), few atrophic tubules, few granular and hyaline casts, and calcification in both specimens. The interstitium showed mild patchy lymphocytic infiltration and fibrosis. The test for determination of urine oxalate level was not available. The parents refused genetic tests for their child. Despite supportive therapies and correction of fluid and electrolyte abnormalities, the case gradually became oliguric progressing to anuria, and was placed on peritoneal dialysis.
3. Discussion
The current study presents primary hyperoxaluria in a male infant with high serum creatinine level and many calcium oxalate crystals in both kidneys. Primary Hyperoxaluria are rare autosomal recessive diseases caused by defects in glyoxylate metabolism, characterized by systemic oxalosis, highly elevated urinary oxalate, resulting in recurrent urolithiasis and/or progressive nephrocalcinosis that eventually causes end-stage renal disease (ESRD) (1, 16).
Primary Hyperoxaluria type I is the most common (70% to 80%) and the most severe form of PH and manifests during childhood with recurrent kidney stones and nephrocalcinosis. Primary Hyperoxaluria-II is less common (approximately 10%) with lower urinary oxalate levels and better renal outcomes than PH-I. Patients with PH-III are less likely to develop ESRD than patients with the other two types (1, 2, 15).
Once the calcium oxalate crystals adhere to the cell, they are internalized, make changes in gene expression, cause cytoskeletal reorganization, and possibly fibroblast proliferation (1, 15). Inflammasome is considered of major importance in the loss of kidney function, emphasizing the importance of anti-inflammatory therapies to slow the progression of nephropathy in inherited and acquired forms (17).
By light microscopy, calcium oxalate crystals are gray-white, speculated and are birefringent under polarized light, whereas calcium phosphate crystals do not polarize (5, 7). Few or isolated tubular oxalate crystals are not rare in normal or failing kidneys, and in transplanted kidneys; their presence does not imply renal damage. On the contrary, abundant tubular or interstitial calcium oxalate deposits are highly suggestive of a hyperoxaluric condition (7).
Patients with oxalate disorders present different signs and symptoms; the first sign or symptom is usually hematuria or dysuria, urolithiasis, or infection. Renal failure in “infantile oxalosis” presents failure to thrive, anemia, and acidosis. The majority of patients are symptomatic before 10 years of age. In some cases, however, the disease may persist unrecognized until adulthood (1). In systemic disease, calcium oxalate crystal deposits within different tissues, including bones, joints, heart, eyes, vessel walls, skin, and the central nervous system (1, 15).
Lack of awareness of PHs and their heterogeneous clinical presentations may obscure the diagnosis until the disease is advanced (2). Early intervention can delay or even prevent ESRD. Transplantation methods depend on the type of PH; combined liver and kidney transplantation is preferred in PH-I and isolated kidney transplantation is the method of choice in PH-II. Progression to ESRD has not been reported in PH-III (1).
Calcium oxalate stone formation during childhood or adolescence, and recurrent stones, multiple stones, or nephrocalcinosis in patients with renal failure are important diagnostic clues that suggest metabolic screening and, if necessary, specific diagnostic testing (2).