1. Background
Cystic fibrosis (CF) is the most prevalent autosomal recessive disease in Caucasians (1); however, no data regarding the prevalence of CF in Iran is available. Although the genetic defect responsible for the development of CF is still unknown, the association between abnormal cystic fibrosis transmembrane conductance regulator (CFTR) protein and chronic pulmonary diseases is becoming more apparent (2).
Chronic respiratory infections that may occur at the early stages of life are among the main symptoms of CF. Pulmonary infections are the most common cause of morbidity and mortality in children with CF and result in early death of 90% of CF patients (2). In studies conducted in the last 3 decades, Pseudomonas aeruginosa was the most frequent microbial agent detected in the airways of CF patients. Bacterial infections are the most common cause of morbidity and mortality in this group of patients (1). Various microorganisms comprise the microbial flora of the airways, the most important of which are Staphylococcus aureus, P. aeruginosa, and Haemophilus influenza. The percentage of colonization of the aforementioned microorganisms in children aged 6–10 years was 60%, 40%, and 25%, respectively (1). In recent years, the incidence and prevalence of infections with methicillin-resistant S. aureus (MRSA), Stenotrophomonas, Burkholderia cepacia, Alcaligenes xylosoxidans, Klebsiella species, and nontuberculous mycobacterium (NTM) have increased (3). Among these pathogens, B. cepacia is especially important because it can cause severe respiratory failure and eventual death (4). Moreover, these bacteria can spread among patients and cause epidemics in CF centers (5). The prevalence of B. cepacia in CF patients in Germany in the year 2000 was 2.4% (6). Additionally, the percentage of colonized patients increases with age and severity of disease. The results of previous studies suggest that antibiotic prophylaxis in patients with respiratory infections can increase forced expiratory volume in 1 second (FEV1) values and decrease the duration of hospital stay (7, 8). Researchers have also shown that maintenance therapy with antipseudomonal antibiotics in children with CF can improve pulmonary function test (9).
2. Objectives
This study aimed at evaluating the clinical findings of laboratory tests, bacterial colonization, and antibiotic resistance in children with CF who hospitalized at the Pediatric Pulmonary Department of Masih Daneshvari Hospital.
3. Patients and Methods
This cross-sectional study was conducted using the medical records of 23 patients with CF who were hospitalized at the Pediatric Department of Masih Daneshvari Hospital in Tehran, Iran, between 2006 and 2010. All patients with a positive sweat test (chloride level > 60 mg/L) and clinical symptoms indicating CF were included in the study as a convenient sampling manner. Information regarding age, sex, family history of CF, consanguinity of the parents, and laboratory test results (including sputum culture and microbial antibiotic susceptibility/resistance test results) collected from the patients’ medical records. Sputum culture was performed on MacConkey agar, chocolate media, and blood agar. Next, the disc diffusion method was used for detection of microbial antibiotic susceptibility. Antibiograms were obtained using cotrimoxazole, ceftriaxone, ceftazidime, amikacin, and ciprofloxacin kits (Darvash Company and Padtanteb Company) (these kits were selected because of the limited variety of kits available in the referral laboratory). Data were analyzed using SPSS version 11.5. Prophylactic treatment was administered to all patients after discharge from the hospital. ANOVA and t tests were used to evaluate the relation between variables; normality of numeric variables in different states of categorical variables was assessed with K-S test. P values less than 0.05 were considered statistically significant.
4. Results
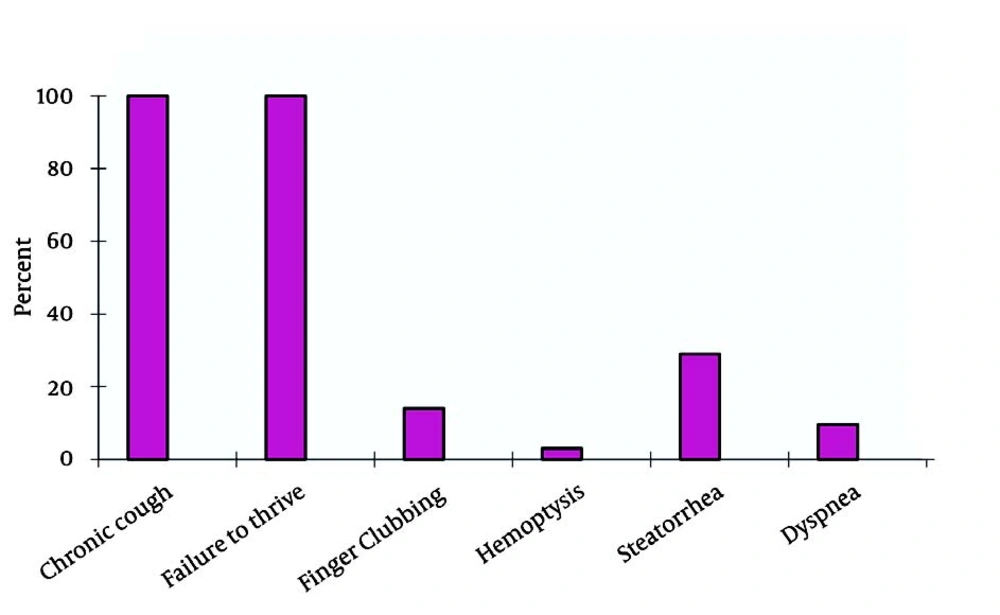
The 23 CF patients included 12 female (52.2%) and 11 male (47.8%). The mean age of the patients was 14.5 ± 6.7 years. The mean age of the patients at the time of diagnosis was 9.5 ± 7.3 years. A family history of CF was found in 1 (4.3%) patient. Parental consanguinity was noted in 16 (69.6%) (95% CI, 50.8-88.4%) of the cases. In 18 (78.3%) (95% CI, 61.4-95.1%) of the patients, growth indices were below 10%. In 3 (13%) (95% CI,0-26.8%), these figures were below 3%. Evaluations showed that the most common complaints were productive cough, growth retardation, and gastrointestinal complications (Figure 1).
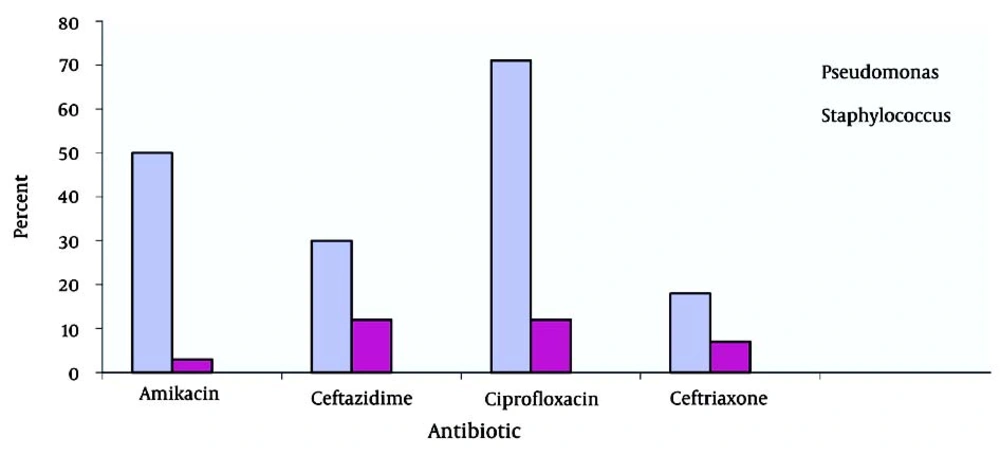
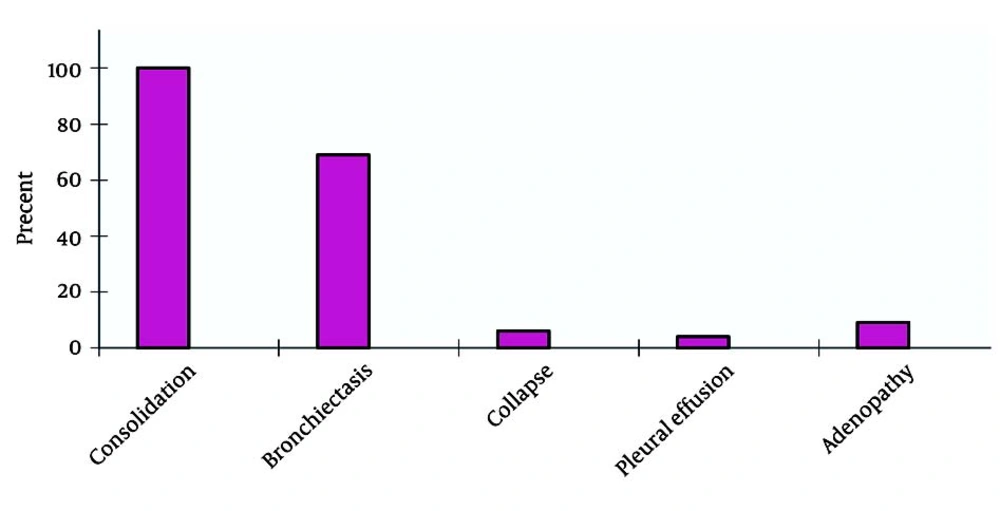
Six-minute walk tests yielded abnormal results in 22 subjects. Pulmonary function tests showed a severe restrictive pattern in 8 (34.7%) patients and a severe obstructive pattern in 3 (13%) patients. Of the 23 patients, 10 (5 male and 5 female patients) had a positive sputum culture for P. aeruginosa and 2 (1 male and 1 female patient) had a positive sputum culture for S. aureus. Positive sputum culture for NTM was observed in 2 patients. TST (tuberculin skin test) yielded negative results in 12 female (55%) and 10 male (46%) patients. In the present study, the highest susceptibility levels were recorded for ciprofloxacin (71.41%), amikacin (50%), ceftazidime (30%), and ceftriaxone (18%) (Figure 2). Hepatic cirrhosis, cor pulmonale, and acute appendicitis (which necessitated appendectomy) were detected in 2 (6%), 4 (18%), and 2 (6%) cases, respectively. One patient had a history of pulmonary tuberculosis (3%). After diagnosis of CF, prophylaxis with nebulized antibiotics, chest physiotherapy, and supplementary vitamins were prescribed for all patients. According to the medical records of the patients, 13 patients received regular follow-up, whereas 9 patients did not. Statistical comparison between the 2 groups (patients who underwent follow-up and those who did not) revealed a significant decrease in the number of respiratory infections per year in those who were receiving regular prophylaxis (P < 0.05). Chest CT scan findings are summarized in Figure 3.
5. Discussion
The lungs of CF patients are colonized with various microorganisms. In these patients, airway inflammation can start at an early age. Epidemiological studies have not been able to elucidate the routes of transmission and prevalence of cross-infections. At present, the main clinical problem in CF patients is airway infection with P. aeruginosa and associated inflammation. In this study, the main pathogen in patients with chronic pulmonary infections was P. aeruginosa. Although treatment and antibiotic prophylaxis have decreased morbidity and mortality in CF patients, the problem of resistance to antibiotics for P. aeruginosa still exists (9-12). Despite treatment, P. aeruginosa can remain in the lungs of CF patients and lead to serious problems.
The role of inflammation in destroying tissue and the resultant dysfunction and failure of the organ has been shown in a number of studies (13). Use of anti-inflammatory treatment in infectious patients can result in clinical recovery or improvement (14, 15). Various studies have shown that inflammation and bacterial infection occur at an early age, before the manifestation of the main clinical symptoms. The results obtained by Flume et al. for the clinical symptoms of CF (13) are in agreement with those presented here. Bronchiectasis causes focal hemorrhagic pneumonia, which can explain the occurrence of bloody sputum in CF patients (14). Chest CT scan findings show that 48% of patients develop bronchiectasis (15). In fact, CF is among the main causes of bronchiectasis. In a study performed in 1998, Coming et al. showed that in 39% of bronchiectatic patients, CF was the main cause (15). In CF patients, accumulation of purulent mucous secretions in the airways and destruction of lung parenchyma results in the development of cor pulmonale (16). This condition was noted in 3% of the cases assessed in this study.
One of the important complications of CF is the development of recurrent respiratory infections. Administration of antibiotic prophylaxis is an effective method for decreasing the prevalence of pneumonia in CF patients (17). The findings presented here show that proper antibiotic prophylaxis could decrease the prevalence of pneumonia and reduce the number of annual respiratory infections in CF patients. In a study conducted in the US on 520 CF patients, administration of tobramycin significantly decreased the hospitalization rate (18). Another study showed that use of inhaled tobramycin (2-3 times per day) in CF patients was associated with improved pulmonary function and decreased number of PA (P. aeroginosa) in the sputum of these patients (19).
The presence of NTM in CF patients is being increasingly recognized. This may be due to high environmental exposure to NTM and the rapid progression of the disease. In this study, 2 of the 23 patients had positive cultures for NTM (MAC) (mycobacterium avium complex). We recommend that in patients who are not responding to the usual CF treatment, the presence of NTM should be evaluated (20).
It is generally believed that long-term use of oral or inhaled antibiotics can suppress respiratory infections (21). In Iran, patients mostly use nebulized gentamycin or amikacin with azithromycin every other month because the cost of inhaled tobramycin is high. This method although decreases the number of microorganisms, can be associated with drug-induced complications or development of drug resistance. The results of this and other studies suggest that proper administration of prophylactic antibiotics (especially in children with positive culture for P. aeruginosa) along with inhaled medications, vitamins, and chest physiotherapy may decrease the disease prevalence and reduce the rate of complications and frequency of hospital stays in children with CF (22). In a study conducted by Anthony and colleagues in 2002 on 140 children with CF, H. influenza and B. cepacia were found to be the most prevalent colonizing microorganisms in the airways (75% with positive culture). Their results are comparable with the results presented here, which showed that 43% and 8.6% of cultures in this study were positive for P. aeruginosa and S. aureus, respectively (23). In a multi-center study conducted by Eftekhar and coworkers on 64 CF patients in Tehran, P. aeruginosa was reported in 21 cases (6 cases of mucoid and 15 cases of classic) (24). In the current study, the highest antibiotic susceptibly was to ciprofloxacin (71.41%), amikacin (50%), ceftazidime (30%), and ceftriaxone (18%).
Considering all the above factors, it can be concluded that the lungs in CF patients are ideal environment for the growth of pathogenic microorganisms. As the number of drug-resistant colonies of pathogens increases, treatment of infections in CF patients becomes more challenging. However, use of vaccines and inhaled antibiotics as prophylactic agents could be beneficial in this respect.