1. Background
Primary immunodeficiencies (PIDs) are a rapidly developing part of modern medicine (1). Today, more than 200 primary immunodeficiencies have been described and characterized (2, 3). Due to the heterogeneity of the PIDs, the variability of their clinical manifestations, and involvement of different organs, a multidisciplinary approach is required. The early diagnosis and initiation of appropriate therapeutic strategies will significantly influence the prognosis. PIDs are usually rare diseases with overall prevalence of 1:10000 live births (4). PID encompasses a wide spectrum of manifestations ranging from selective IgA deficiency with a considerably benign prognosis to severe combined immunodeficiency (SCID) with a potentially catastrophic outcome. Increased susceptibility to infections is the hallmark of these disorders. Furthermore, dysregulation of the immune system may predispose these patients to malignancy and autoimmune disorders (5).
For proper management of these rare conditions, epidemiological data are of paramount importance in making health-care planning decisions. However, due to rarity of these disorders, and the existing differences in incidence of PIDs in certain geographic regions of the world, epidemiologic data on PID are difficult to obtain. Therefore, PIDs are often under-diagnosed and under-reported. In addition, data on mortality and morbidity are limited. This study aims to provide data on different aspects of PIDs in the children age group presented to Mofid University Hospital, Shahid Beheshti University of Medical Sciences from 2003 to 2013.
2. Methods
This study included all the patients referred to infectious and immunology clinics of Mofid University Hospital during a 10-year period from 2003 to 2013 with clinical presentations indicative of immune deficiency syndromes. The patients were carefully evaluated with a detailed history, physical examination, and immunological screening tests. CBCs were performed by automated blood counting machine, the Sysmex XE-2100. ESRs were measured by Western Green Method. Hemagglutinins were quantified by CA 1600 and immunoglobulin levels were evaluated by ELISA. Reviewing of CD markers was performed by flow cytometry and the level of serum complement components were determined by ELISA. Then, the patients were categorized based on the primary presentations, clinical manifestations of the disorders, and also the laboratory data. The complete blood count of the patients were interpreted according to the reference tables in Nelson Textbook of Pediatrics (6).
Patients with HIV infection, receiving immunosuppressive therapies, and organ transplantations were excluded from the study. A total of 80 patients were enrolled, however, 8 were lost to follow-up. Once phenotypic data was collected from the patients, a genetic pedigree analysis was performed, which allowed us to determine whether the trait was recessive or dominant and to evaluate the risk of repeated occurrence of the disease in the family with performing consultations for future pregnancies. Regular follow-up visits in the clinics was recommended to all the patients.
Before enrollment, a written formal consent was obtained from the patients’ parents or their care-givers. This study was approved by the Ethics Committee of the Mofid Hospital, Shahid Beheshti University of Medical Sciences.
Data were analyzed by statistical software SPSS-19. The level of significance was considered P < 0.05. The Kolmogorov-Smirnov test was used to compare the samples with a reference probability distribution. In the cases with normal parameters, Pearson’s Chi-square test was used. Otherwise, Spearman’s rank correlation coefficient was used as a nonparametric measure of statistical dependence between the variables.
3. Results
In this study, 80 patients were evaluated of whom, 49 (61.2%) were male and 31 (38.8%) were female. The patients’ mean age at onset of presentation was approximately 12 months and their mean age at diagnosis was 23 months. Therefore, the median interval between the onset of symptoms and the diagnosis of a PID in this study was 11 months. The mean age at the onset of symptoms and the mean age at diagnosis according to specific defects are demonstrated in Table 1. As shown in Table 1, the youngest patients with primary immunodeficiencies were those presenting with leukocyte adhesion defect. The mean age at onset of the clinical manifestations for leukocyte adhesion defects in this study was 2.22 months, followed by transient hypogammaglobulinemia of infancy, which was 4.22 months.
| Disorder | Mean Age at Onset, mo | Mean Age at Diagnosis, mo |
|---|---|---|
| SCID | 3.33 | 9.85 |
| Ataxia-telangiectasia | 20.8 | 26.2 |
| CGD | 12.92 | 39.2 |
| IgA deficiency | 13 | 15.2 |
| CVID | 25.18 | 38.42 |
| LADs | 2.22 | 10.57 |
| Bruton | 8.46 | 27.3 |
| Cyclic neutropenia | 6.7 | 15.75 |
| Hyper igE | 15.07 | 32.38 |
| Wiscott-aldrich syndrome | 6.73 | 11 |
| THI | 4.22 | 13 |
| CID | 22.6 | 34.2 |
The Age at Onset of Presentation Versus the Age of Diagnosis in Patients Presenting with Primary Immunodeficiencies
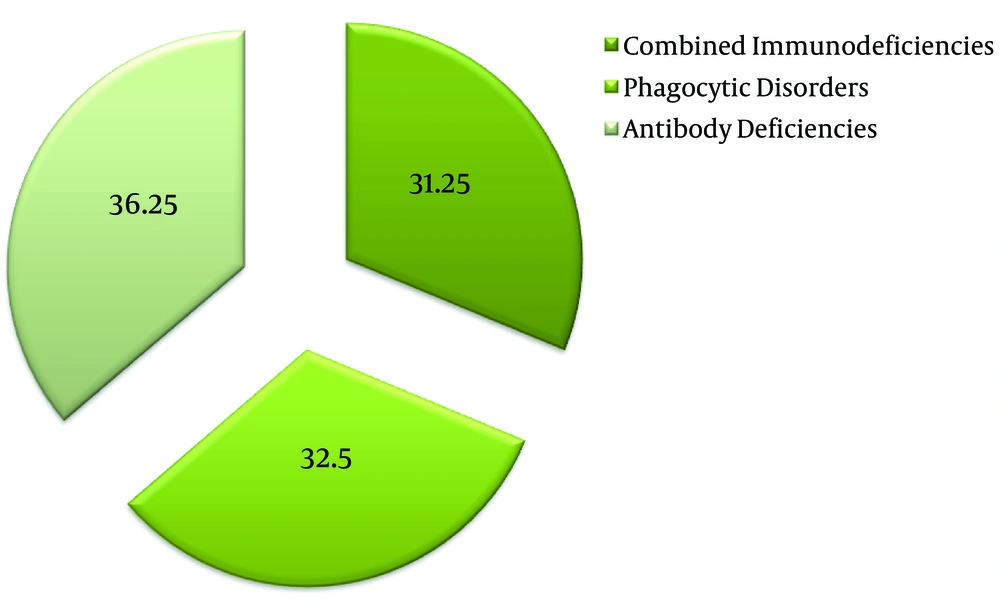
Figure 1 demonstrates the distribution of the immunodeficiencies among the studied population. A total of 29 of our patients (36.5%) were suffering from humoral immunodeficiencies, 26 of them (32.5%) were suffering from phagocytic defects, and 25 patients (31.25%) had combined immunodeficiencies. Table 2 demonstrates the number of patients presenting with specific immunodeficiencies.
| Diagnosis | Number of Patients | Percent of Patients |
|---|---|---|
| CVID | 11 | 13.75 |
| SCID | 10 | 12.50 |
| XLA | 10 | 12.50 |
| CGD | 8 | 10 |
| LADs | 7 | 8.75 |
| Hyper igE | 7 | 8.75 |
| WAS | 6 | 7.5 |
| Selective igA deficiency | 5 | 6.25 |
| CID | 5 | 6.25 |
| Ataxia-telengiectasia | 4 | 5 |
| Cyclic neutropenia | 4 | 5 |
| THI | 3 | 3.75 |
The Number of Patients Presenting with Specific Primary Immunodeficiencies
We sought the most common infectious complications in our patients. Respiratory tract infections were the first warning sign detected in these patients. The most frequent respiratory presentations were pneumonia (30%), otitis media (18.8%), and sinusitis (14%). Other non-specific respiratory signs or symptoms detected in these patients were dyspnea (27.5%), respiratory distress (11.3%), and postnasal drip (8.8%).
We also evaluated the gastrointestinal system in these patients. The most common findings were diarrhea (15%), gastroesophageal reflux (11.25%), and hepatosplenomegaly (11.25%). However, we also detected abdominal pain (22.5%), nausea (8.75%), and vomiting (13.75%) as nonspecific symptoms in these patients.
We evaluated leukocytes and their differential counts in these 80 patients. A total of 63.81% of the patients had normal leukocyte counts. Among the remaining patients, leukopenia was more common (24.91) than leukocytosis (11.25%). Neutropenia in these patients was more frequent in those with combined immunodeficiencies (38.46%), comparing to humoral (34.4%) and phagocytic immunodeficiencies (34.6%). Eosinophilia was another finding, which was more frequent in patients with humoral immunodeficiencies (34.7%). Lymphopenia was more common in combined immunodeficiencies (26.9%). Anemia was found in 60.86% of patients with phagocytic disorders, 57.69% of combined immunodeficiencies, and 52.17% of antibody immunodeficiencies. Thrombocytopenia was found in 42.3% of patients with combined immunodeficiencies, in 30.43% of patients with humoral immunodeficiencies, and 13.04% of patients with phagocytic disorders.
Parental consanguinity was the next variable evaluated in these patients. A total of 59.25% of parents were first cousins and 3.7% were double first cousins. A total of 11.14% of the parents had a nonconsanguineous marriage. We reviewed the pedigree of these patients, and found out that the disorder had been repeated in 11% of cases previously in the family pedigree, however, the majority (89%) of the pedigrees did not demonstrate any similar cases in other descendants. The mode of inheritance of the immunodeficiency disorders were mostly autosomal recessive (88.88%), followed by autosomal dominant, and X-linked recessive (each 3.7%). A total of 3.27% of the cases were sporadic
4. Discussion
More than 200 immunodeficiencies have been detected so far and new PIDs are being discovered at an increasing rate. PIDs are rare, however, they comprise diverse genetic defects with adverse influences on the development and function of immunity (7). They result in a wide range of clinical symptoms, including susceptibility to infections, auto-inflammation, allergy, and malignancy. The early diagnosis is usually associated with better prognosis and sooner establishment of appropriate therapeutic strategies. Besides, proper management may prevent or slow down the development and course of the complications of PIDs, particularly the respiratory consequences. Diagnosis is based on immunologic tests and molecular genetic analysis. In some countries, newborn screening programs are running with the aim of early initiation of appropriate treatment and to prevent the possible complications of these diseases (8).
This study was conducted to evaluate the epidemiologic characteristics of primary immunodeficiency disorders in patients attending Immunodeficiency Clinic of Mofid University Hospital, Shahid Beheshti University of Medical Sciences.
As mentioned, in previous sections, male predominance was evident in this study. In other studies, the overall incidence of immunodeficiency diseases was reported 1.4 to 2.3 times more common in males than in females (9). This figure in our study was approximately 1.5. This male predominance is true even if X-linked diseases are excluded. The reason is unknown, however, it may indicate additional genetic susceptibility factors. Boys were also affected by autosomal disorders due to the high rate of parental consanguinity, which was significantly high in these patients (89%).
The age at onset of symptoms, age at diagnosis, and the diagnosis lag showed considerable variations among different primary immunodeficiencies in the patients evaluated. However, the median interval between the onset of symptoms and the diagnosis of PID in this study (11 months) seems to be an acceptable period of time. Nearly, the same results were obtained in another population-based cohort study (10). The patients’ mean age at the onset of symptoms in this study was approximately similar to other studies performed earlier.
Evaluation of these patients revealed that B-cell defects predominated in the patients. However, considering all of the patients in our study, the two most common primary immunodeficiencies were common variable immunodeficiency and severe combined immunodeficiencies (12.5%). Ataxia-telangiectasia (5%), cyclic neutropenia (5%), and transient hypogamaglobulinemia of infancy (3.75%) were among the primary immunodeficiency disorders with the lowest prevalence in our study. The same results have been obtained in other studies. For instance, common variable immunodeficiency was the most common entity in the European Internet based patient and research database for primary immunodeficiencies (9) with 2880 patients or 21% of all the patients studied. This was also true in a study conducted by Stray-Pederson et al. in Norway (11). Our findings were compatible with these studies, although the studied population was much smaller than the above studies.
We also evaluated the associated morbidities in these patients to detect the most frequent signs and symptoms. Recurrent pneumonia (28.8%) was the most common initial manifestation, followed by recurrent ear infections (18%) and recurrent or chronic sinusitis (14%). Al-Herz et al. also showed that pneumonia was the most common infectious complication (60%), however, in other studies the rate of pneumonia varied between 23% to 42% (12). In another study designed by Jung Woo Rhim et al. the most frequent findings in patients with primary immunodeficiencies were pneumonia, otitis, and adenitis (12, 13). Adenitis was also a frequent finding in our study, however, lymph node involvement in our patients particularly suggested phagocytic disorders such as chronic granulomatous disease. Fever was the most frequent constitutional symptom of the patients in this study. Cytopenia was another finding detected in detailed evaluation of our patients. A variety of causes for cytopenia have been proposed in PIDs. In some types of immunodeficiencies, it is a primary feature of the immunodeficiency, and in others, it is a secondary phenomenon. The following pathophysiological mechanisms have been suggested as the cause of cytopenia in immunodeficiency syndromes: (1) classic autoimmune cytopenia, (2) cytopenia in the context of immune dysregulation, (3) bone marrow failure, (4) toxic or infectious myelosuppression secondary or concomitant to PID (14).
WBC counts were mostly normal in nearly all of the patients in our study. Thrombocytopenia was found in 30.43% of patients with humoral immunodeficiencies. The most common reason found, according to the above-mentioned etiologies, was due to antibody-mediated autoimmunity particularly in patients with CVID. Autoimmunity is not exclusively seen in CVID, however, many other disorders such ac CIDs may show some extent of autoimmunity as a result of autoreactive T cells and reduced T cell regulation, which could explain the cause of cytopenia. However, in Wiscott-Aldrich syndrome, the basis of thrombocytopenia is the underlying cytoskeletal dysfunction (14). Anemia was a more common finding in patients with phagocytic disorders (60.86%) as a complication of toxic or infectious myelosuppression.
Eosinophilia was a frequent finding in the patients studied. In addition to the typical PID conditions described with eosinophilia, a wide range of PIDs have been associated with eosinophilia. It has been documented that MHC class II deficiency, CD3γ deficiency, STAT1 deficiency, Kostmann disease, Cyclic neutropenia, CD40 deficiency, CD40L deficiency, anhidrotic ectodermal dysplasia with immune deficiency, ataxia-telangiectasia, CVID, CARD9 deficiency, CGD, MALT1 deficiency, and many other well-known primary immunodeficiencies, have been noted to have elevated eosinophils (15).
Neutropenia is a clinical feature of several primary immunodeficiency disorders. In this study, neutropenia was a common finding with combined immunodeficiencies.
The overall results we found in this study are consistent with previously reported studies in other countries. However, we believe this study is subject to some limitations. First, Mofid Teaching Hospital is a tertiary center and this study was conducted on a small number of patients presented at this tertiary hospital. Therefore, the prevalence of different PIDs cannot be generalized to the whole country. Second, patients were presented at our hospital due to the fact that they either had critical acute illnesses or chronic difficult-to-treat complications. Thus, the study was biased towards the severe complicated forms of PIDs.
4.1. Conclusions
The diagnosis of PID has been increased during the past decades. As noticed, an overall increase in incidence over time could be due to better diagnostic tools and increased physician awareness of these disorders. Such epidemiological data are needed to raise the awareness of the medical community about PIDs and to support the public health benefits of early diagnosis and treatment.