1. Context
Previously, it has been indicated that 24 - 53% of children admitted to pediatric intensive care units (PICUs) suffer from acute or chronic malnutrition at the time of admission (1, 2) and a large number of them undergo a deterioration of nutritional status during hospitalization (2). Malnourished hospitalized patients usually encounter several complications such as infections, increased mortality, prolonged length of hospital stay, and perhaps poor outcome (3). There are several documents proposing that an early nutritional assessment and consequently early intervention can prevent or reduce the complications of malnutrition (4, 5).
Critically ill pediatric patients are at increased risk of malnutrition because of altered metabolism. These changes include increased basal metabolic rate and enhanced protein catabolism (6, 7). The metabolic response to stress in critically ill pediatric patients causes the amino acids of lean tissues to mobilize in order to support accelerated demand for protein synthesis. Supporting the hypermetabolism and consequent high demands for energy, protein, and other nutrients through an early and appropriate nutritional intervention is the ultimate goal of nutritional support therapy (NST). The NST is indicated when a patient is unable to have an adequate intake of calories and nutrients orally for a period and contains two main enteral and parenteral routes.
The careful nutritional evaluation of all patients at the time of admission to PICUs and identification of pre-existing malnutrition are essential in both diagnosis and treatment management of the patients. This baseline evaluation helps us to identify those patients at risk of developing malnutrition or further nutritional deterioration due to their present illness. This assessment also allows early supportive nutritional intervention to optimize the nutrient intake.
In spite of the well-known benefits of nutrition support in hospitalized pediatric patients, the awareness of disease-related malnutrition is inadequate and malnutrition remains a medical problem in many PICU patients in our country, Iran. There are limited numbers of PICU care teams in Iran that have clinical nutritionists as a member. This is because of underestimating the significant role of malnutrition and its consequences by many health care professionals in our country that results in lacking medical awareness of malnutrition in many PICU admitted children.
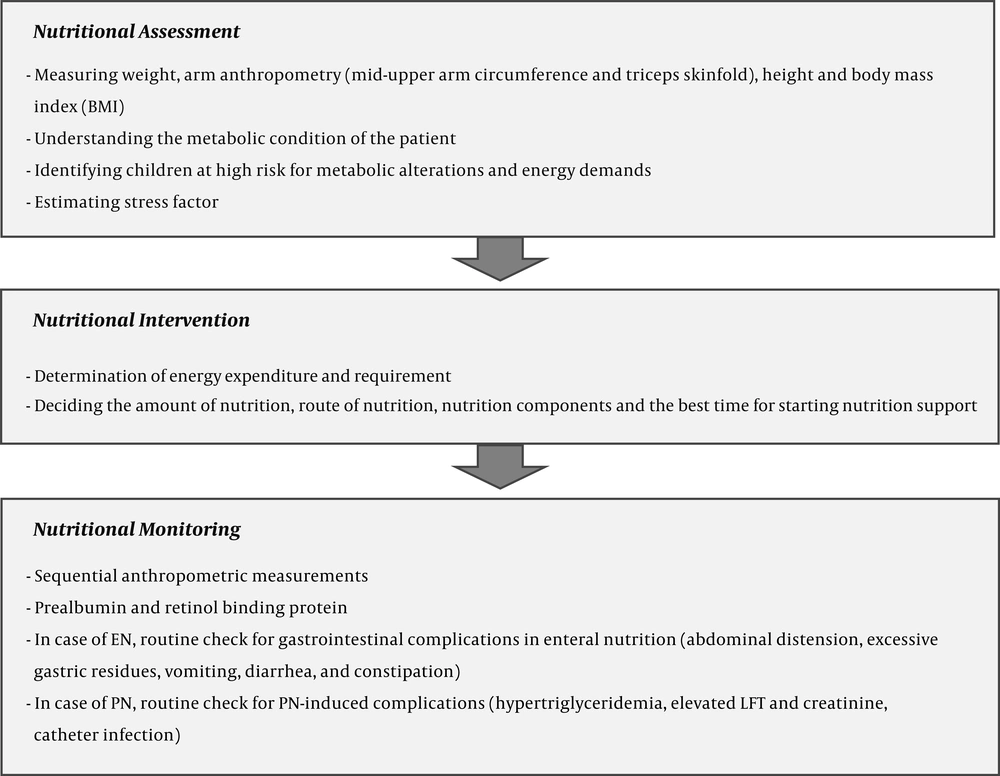
Nutrition therapy in critically ill children briefly includes three steps: Baseline nutritional assessment, nutritional intervention, and monitoring. These three steps are discussed in the current review, aiming to reduce the number of PICU patients who deteriorate mainly due to poor nutritional support.
The purpose of this review is to describe three steps of nutritional management in PICU admitted children and discuss the importance of each step.
2. Evidence Acquisition
This review provides updates on nutritional management in PICU care with a focus on three steps of nutritional assessment, intervention, and monitoring that have been developed during 2002 - 2015 (Figure 1). Papers reviewed were in English. Databases such as PubMed/Medline, Scopus, ScienceDirect, and Google Scholar were investigated based on the combination of the following keywords: ‘Pediatric intensive care unit’, ‘nutrition assessment’, ‘nutrition support therapy’, ‘parenteral nutrition’, ‘enteral nutrition’, and ‘nutrition monitoring’.
The literature search was undertaken to discuss the following topics:
- The best and most practical nutrition screening and assessment methods to be used in pediatric intensive care units.
- Essentials of nutrition intervention and nutritional support in PICU patients.
- Highlights of monitoring and evaluation following a nutrition intervention in pediatric intensive care units.
3. Results
3.1. Nutritional Assessment
Patients admitted to PICUs are at increased risk of anthropometric changes and deterioration in the nutritional status because of altered metabolism, together with increased nutritional needs. Based on previously published guidelines and reviews, nutrition assessment in PICU patients within the first 24 hours of admission or during the course of critical illness is desirable (5, 8-10).
Several indices have been introduced for nutritional assessment. A routine, and probably, easier-to-measure index is weight. When reporting the changes in weight and other anthropometric measurements, the context of fluid therapy, probable causes of volume overload, and diuretics should be considered. In addition to weight, arm anthropometry (mid-upper arm circumference and triceps skinfold), height, and body mass index (BMI) are commonly used (11). Body composition is another parameter of nutritional assessment at admission so that it is a predictor of mortality and morbidity in children (11). Preserving lean body mass during hospitalization has been shown as an important predictor of clinical outcome in situations such as sepsis, cystic fibrosis, and malnutrition (12, 13).
Serum levels of albumin at admission do not reliably reflect the immediate nutrition status. The reason is that albumin has a large pool and long half-life and its serum levels may be affected by albumin infusion, dehydration, sepsis, and liver disease. In addition, prealbumin, which is a negative acute phase protein, cannot accurately reflect the nutritional level and responses to nutritional support during inflammation (14). However, prealbumin is measured in most hospitals as an indicator of more acute nutritional changes (11).
Understanding the metabolic condition of the patient is an important part of the nutritional evaluation. In critically ill children, a catabolic response is induced and the serum levels of counter-regulatory hormones are increased. The former induces insulin and growth hormone resistance, resulting in the catabolism of proteins, carbohydrates, and fats in order to provide energy and substrate for increased metabolic demands (15). A failure to provide adequate energy and nutrients during the hypermetabolic phase may waste the fat-free mass and transfer the patient into a malnutrition phase, which results in several complications. The routine method for adjustment of increased energy demand in measured resting energy expenditure (REE) of a patient is multiplying REE by stress factor, which is determined based on the nature of the illness, its severity, and growth status of the child (16, 17). Besides the several complications of PICU-related malnutrition, overfeeding has been indicated to accompany increased ventilator work and longer need for mechanical ventilation, steatosis, cholestasis, and increased risk of infection due to hyperglycemia (18, 19).
Several screening tools such as Screening Tool for the Assessment of Malnutrition in Pediatrics (STRONGkids), Pediatric Yorkhill Malnutrition Score (PYMS), and Tool for the Assessment of Malnutrition in Pediatrics (STAMP) have been developed aiming at nutritional assessment and risk evaluation in newly admitted pediatric patients that have been translated into several languages (20-22). Some evidence has found a stronger relationship between STRONGkids tool and anthropometric measurements (23, 24).
Children at high risk for metabolic alterations and energy demands should be identified for targeted measurement of REE in PICUs. These children include (14):
1) Underweight (BMI < 5th percentile for age), at risk of overweight (BMI > 85th percentile for age), or overweight (BMI > 95th percentile for age) children.
2) Children with > 10% weight gain or loss during PICU stay.
3) Children failed to consistently meet the prescribed caloric goals.
4) Children failed to wean or in need of escalating respiratory support or requiring mechanical ventilator support for > 7 days.
5) Children suspected to be severely hypermetabolic (status epilepticus, hyperthermia, systemic inflammatory response syndrome, dysautonomic storms, etc.) or hypometabolic (hypothermia, hypothyroidism, pentobarbital or midazolam, etc.).
3.2. Nutritional Intervention
Another step, next to identifying patients at risk of malnutrition, is nutritional intervention. It has been documented that malnutrition is preventable in critically ill pediatric patients. This step starts with the determination of energy expenditure and requirement that is the most appropriate route for administration of nutrients in the child.
Energy requirement in PICU-admitted patients is usually calculated using anthropometry indices and stress equations based on critical illness and nutrition status of the patients. The use of equations has been shown to be inaccurate and underestimate or overestimate the real nutritional needs in some situations (25). Indirect calorimetry (IC) is the most accurate method for calculating the energy expenditure in patients. Limitations in equipment availability, staffing, and costs restrict the routine use of IC even in equipped PICUs and thus, most European centers report the use of equations for estimation of energy expenditure rather than accurate measurements in critically ill patients (26). Indirect calorimetry reflects resting energy expenditure (REE) by measuring the respiratory quotient (RQ), which is the ratio of carbon dioxide produced (VCO2) to oxygen consumed (VO2). Underfeeding, which results in the use of endogenous fat stores, and overfeeding, which promotes lipogenesis, can cause RQ to decrease and increase, respectively (27).
In PICU-admitted patients, who have an intact and functioning gastrointestinal tract, the enteral route is preferred. In those patients who cannot receive adequate requirement through enteral route alone, the parenteral route is also added (28, 29). The enteral nutrition (EN) maintains the integrity of enterocytes, reduces the risk of sepsis, and is well tolerated even in patients who receive vasoactive drugs, which may reduce intestinal perfusion (30, 31). Adhesion to guidelines for nutritional support in PICU patients has been along with earlier initiation of EN, decreased length of hospital stay, shortened time to reach a caloric goal, and decreased EN interruptions (32-34). The EN should be initiated immediately when the gastrointestinal system becomes receptive. An earlier establishment of EN could reduce the incidence and severity of cholestasis and other complications related to PN (35). However, a systematic review on 2013 points out that scientific evidence on the use of early enteral nutrition therapy in improving the development of critically ill pediatric patients is still scarce and further studies are needed focusing on it (36).
There are insufficient data concerning the preferred site for enteral tube placement. One randomized controlled trial on 2004 compared gastric and post-pyloric feeding sites and reported a better tolerance in patients who received early (< 24 hours after PICU admission) vs. late (started after 24 hours) post-pyloric enteral feeding (30). This option can be considered in those patients who relatively cannot tolerate gastric feeding or are at risk of aspiration (14). This is because post-pyloric feeding reduces the volume of gastric residue, microaspiration, and the number of feeding interruptions (37, 38). Furthermore, there is insufficient evidence to support the use of prokinetic medications or motility agents for EN intolerance, as well as prebiotics, probiotics, and symbiotic for children in critically ill situations (14).
Whenever EN is impossible, inadequate, hazardous, or not tolerated, parenteral nutrition (PN) is used (39). The most common reasons that make the enteral feeding intolerable are fluid restriction, interruptions for procedures, and hemodynamic instability (14). Aiming to improve nutrition support, a mixed EN/PN may be performed in a number of patients.
PN is a complex therapy with significant adverse effects. The appropriate and safe ordering of PN is a critical decision so that the American Society of Parenteral and Enteral Nutrition (ASPEN) strongly recommends developing written policies and procedures for all aspects of PN therapy besides a comprehensive PN education for healthcare professionals who are involved in ICU care (35). Even more, the ASPEN strongly recommends using a standardized PN order format like a computerized prescriber order entry (CPOE) system or if not applicable, a standard order template for PN prescription. This order shall contain three components: Patient information, PN detailed ingredients, and PN instructions. Regarding legal considerations, handwritten, verbal, or telephone orders for PN should be avoided (35). PN orders should be clear and precise, as well.
3.3. Nutritional Monitoring
Nutritional intervention must be followed by nutritional monitoring during hospitalization. This is done by sequential anthropometric measurements. The purpose of monitoring is to maintain the nutritional status in addition to evaluate the adequacy and efficacy of the nutritional intervention. Although anthropometric methods (weight and skinfold thickness) are relatively insensitive to short-term changes and several factors such as edema and ascites affect the accuracy of measurements and make the judgment sometimes difficult, anthropometry is still considered as the easiest method for nutritional monitoring (40). Prealbumin and retinol binding protein are serum proteins with a short half-life and are more sensitive to nutritional status, but the application of these indicators in critically ill children needs to be approved (41).
Since PN is associated with some complications, it should be closely monitored. The most highlighted complication of PN is the increased risk of infection (42). The others include hypoglycemia, hyperglycemia, hypertriglyceridemia, metabolic bone disease, and PN-associated liver disease (PNALD) (35). Therefore, the following items should be considered for monitoring: Weight and intake/output (daily), biochemical factors such as electrolytes, glucose, cell blood count (CBC), prothrombin time (PT), albumin, creatinine, liver function tests, triglyceride (daily until the patient becomes stable and weekly thereafter) and nitrogen balance (when needed) (43). Enteral nutrition should also be monitored for gastrointestinal complications, such as abdominal distension, excessive gastric residues, vomiting, diarrhea, and constipation that are most common (44, 45). These complications usually occur in conditions such as shock, acute renal failure, hypophosphatemia, and the administration of catecholamines, sedatives, and muscle relaxants (41). Serial nutritional evaluations allow early detection of nutritional deficiency and indicate the efficacy of nutritional support in PICU patients.
4. Conclusions
Giving the fact that malnutrition during hospitalization in children often remains unrecognized by healthcare workers and a large number of PICU admitted patients undergo a deterioration in nutritional status during hospitalization, considering a stepwise approach toward nutrition management in PICUs might be so useful. The assessment of nutritional status, identifying patients at risk of further nutritional deterioration, accurate measurement of energy and nutrient need for each patient, initiating nutrition support based on NST approved protocols, and regular assessment of anthropometry and nutritional parameters during the admission period are the stepwise phases of nutritional management in PICU patients. Future interventional research such as PEPaNIC (Early Versus Late Parenteral Nutrition in the Pediatric Intensive Care Unit) study can have new clinical messages for improving nutritional status in hospitalized critically ill children.