1. Background
Hyperbilirubinemia is one of the most common complications of neonates, especially among the Asian races. Approximately 60% of neonates, with a gestational age of 35 weeks, show hyperbilirubinemia three or four days after birth. In addition, this disorder can be observed in 50% to 60% of preterm neonates (1-4). Hyperbilirubinemia is one of the most common causes of readmission infants after being discharged from the hospital (3, 5). Total serum bilirubin (TSB) levels increases In hyperbilirubinemia (6, 7).
In normal conditions, the mean of indirect bilirubin in the cord blood is 1 - 3 mg/dL, which increases with a rate less than 5 mg/dL in 24 hours, and the total bilirubin level is 0.3 to 1 mg/dL. Physiological hyperbilirubinemia occurs when the mean of indirect bilirubin achieves 10 - 12 mg/dL in term neonates and 15 mg/dL in preterm neonates (8).
Producing bilirubin is the result of lysis of RBC, releasing of hemoglobin and biliverdin catabolism, which is controlled by the heme oxygenase enzyme (9). Excretion of bilirubin included absorption of bilirubin into the hepatocyte, and then transformation to the conjugated bilirubin. Newly formed conjugated bilirubin is voided of the intestine and bile through the stool and urine. A three-step process occurs in the intestinal flow in infants. The conjugated bilirubin is converted to the unconjugated form, and is then reabsorbed and leaded into the hepatic bilirubin pool through a port flow path for discharge. TSB stabling depends on the balance in the metabolic pathways. Hyperbilirubinemia occurs due to an imbalance in the production and excretion of bilirubin (6, 7).
Hyperbilirubinemia starts first in eyes, then it achieves on the face and spreads to the chest, abdomen, and legs (10). It is particularly important, due to disorders such as kernicterus, surfactant blocking, and increased hemolysis (6, 7, 11).
Considering complications of hyperbilirubinemia, checking of the bilirubin concentration is necessary to diagnose the disorder faster. The golden standard for diagnosis of hyperbilirubinemia is the investigation of TSB (9). However, this procedure is invasive and stressful for neonates. This procedure increases the risk of infections in the site of sampling and possibility increases osteomyelitis (3).
For two decades transcutaneous bilirubin (TCB) measurement was used as a non-invasive, safe, painless, and convenient method to assess total bilirubin and screen the neonates with hyperbilirubinemia, as well as used before, during, and after phototherapy (1, 2, 12). In addition, TCB reduces the frequency of invasive blood samplings, and results of bilirubin concentration are obtained quickly (13, 14).
The TCB is appropriate to investigate bilirubin in term and post-term neonates with hyperbilirubinemia. A high correlation has been reported between the TCB and the TSB, with the exception of black race neonates (15).
TCB was introduced by Yamanouchi et al., as a noninvasive procedure for evaluating the concentration of bilirubin for first time. Yamanouchi et al. constructed the light and portable device for the measuring of hemoglobin (9, 16).
The use of this device was limited at first due to the unreliability of old devices; however, in the second line of providing the device, the accuracy of TSB improved by using wavelength spectral reflectance techniques, in term and preterm neonates (17). This device checks the measurement of yellow color in the blanched skin and subcutaneous tissue (4).
The results of Mansouri et al. showed that TCB correlated significantly with TSB (r = 0.89). They believed that there is a low difference among BiliChek device types, skin color, weight, and race, which can cause an effect on the results of the correlation between the TSB and the TCB measurements (18).
Radfar et al. found a strong correlation coefficient between TCB and TSB prior to the initiation of phototherapy (r = 0.929, P < 0.001). This correlation was significantly weaker after phototherapy (r = 0.921, P < 0.001). They reported that the equilibration process would be delayed with regard to the skin after phototherapy (1).
In the study of Ercan and Ozgun the TSB level of at least 2.5 mg/dL, using a cut-offTCB of 2.3 mg/dL was investigated. Sensitivity was calculated as 95.9% and specificity (59.4%) with a negative predictive value (NPV) of 90% (2).
2. Objectives
Due to the necessity of hyperbilirubinemia early diagnosis, noninvasive, and simplicity of the TCB method for measuring bilirubin concentration, this study was implemented to compare the accuracy of TCB versus TSB before and after phototherapy and determination of the sensitivity and positive predictive value (PPV) of TCB.
3. Methods
3.1. Study and Samples
This descriptive-analytical study was carried out at the neonatal unit of Imam Ali hospital in Zahedan, Iran, in 2015. Research samples were admitted from October 2014 to June 2015. The subjects were selected by convenience sampling methods. Thus, all neonates who were eligible for inclusion in the study were selected gradually until reaching the desired number.
The inclusion criteria were as follows: (1) normal birth weight (2500 - 4000 g), (2) physiological jaundice, (3) breastfeeding, (4) no need for blood transfusions, and (5) lack of respiratory failure and infection.
The exclusion criteria were as follows: (1) symptoms of neonatal sepsis, respiratory failure, and infection, (2) the dissatisfaction of a mother, and (3) discharge from the hospital.
The sample size was calculated according to similar studies, and by calculating the 5% significance level, a power of 80%, and probability of 5% samples abscission, the sample size was estimated as follows (11):
First, 87 neonates were recruited; however, two neonates were discharged with parental consent. Finally, the total samples size were 85 neonates.
3.2. Measurement
A demographic questionnaire, non-citrated tube for TSB measurement, and BiliChek device (APEL, Japan) were used to collect data. Demographic questionnaire had three sections, containing information regarding mother and neonate (age, weight, sex, and…), information on phototherapy, and the concentration of transcutaneous and serum bilirubin.
For the reliability of TCB device, calibration was used. A medical equipment engineer calibrated the TCB device using the standard device to check the reliability. The device error rate was calculated and improved.
Phototherapy devices were made by Makateb Manufacturing Co. (Iran), which had eight lamps with fluorescent blue and optical spectrum between 420 and 480 nm with a useful lifespan of 3000 hours.
3.3. Implementation
The researcher selected the eligible neonates from those admitted to the neonates unit of the Emam Ali hospital, Zahedan, Iran.
The decision to getting a TSB measurement was made by the attending neonatologist. All neonates were naked and diapered to body skin exposure to phototherapy, and eye protection was used during phototherapy. The distance of lamps until the neonates were between 40 and 45 cm. All neonates were turned every two hours from the prone to supine position, and vice versa, to achieve exposure to the light. Environmental lighting was constant during the study period.
Treatment decisions for initiating, maintaining, and discontinuing phototherapy were based solely upon TSB levels. For ethics in research, samples were collected no more than ordered by the physician, and the criteria were the TSB.
In all neonates, the principal researcher (pediatrics nursing practitioner) evaluated TCB. The researcher, simultaneously by checking the TSB (heel stick technique by neonatal nursing staff), pressed the BiliChek three times in the middle of the neonate’s forehead, according to the manufacturer’s guideline, and then recorded the results. In addition, the TCB was measured and recorded in accordance with the above procedure. The mean of these three consecutive times were calculated and compared with the values obtained from the TSB immediately prior to the drawing of the capillary blood for TSB, and before phototherapy TCB was checked.
For the implementation of TSB, neonatal nursing staff obtained serum bilirubin levels via capillary blood samples (heel), and then, TSB was estimated using the acid diazo method. All the TSB samples were sent to the laboratory of the Imam Ali Hospital in Zahedan, Iran, in order to have uniform procedures and eliminate intervention factors.
This approach was done once before the initiation phototherapy, and once 24 hours after the initiation phototherapy. TSB measurement was paired with TCB measurement before phototherapy and after phototherapy.
3.4. Ethical Considerations
The Ethics Committee at Zahedan Medical Sciences University, Zahedan, Iran, approved this study with code: 6025 and conducted it in compliance with all ethical and professional principles. Written and verbal mother’s consent was obtained after adequate explanation to the mothers. The objectives of the study were explained to the mothers and they were assured that this method was safe.
3.5. Data Analysis
Data obtained from the study were analyzed using statistical SPSS version 18 (SPSS, Chicago, IL).
Descriptive statistics were used for demographic data, Chi-square test for qualitative variables, t-test for the comparison of the TSB, and TCB, Pearson, and Linear correlation coefficients to determine the correlation between the TCB and the TSB and ROC curve to assess the value of the cut-off, the sensitivity, and specificity of TCB. The significant level was less than 0.05.
4. Results
4.1. Baseline Characteristics
Findings showed that there were 48 (56.6%) boys and 35 (41.2%) girls. There was no statistically significant difference among neonates in sex (P = 0.15). The mean age was 7.35 ± 3.1 days after birth. The mean gestational age was 38.27 ± 0.49 days and the mean birth weight was 3064 ± 390 g. There was no statistically significant difference among neonates in age and weight (P = 0.45). The correlation between birth weight with the TSB and the TCB was not statistically significant by Pearson correlation test (P = 0.69). No significant difference between gestational age and the TSB and the TCB was found by t-test (P = 0.40).
4.2. TCB and TSB Measurements
Table 1 shows the means of the TSB and the TCB in term of neonates. The mean TCB in neonates with weighing 2500 to 3000 g before phototherapy was (10.14 ± 2.8 mg/dL) and the mean TSB was 8.28 ± 2.93 mg/dL. The significant difference was observed between the mean TCB and TSB before phototherapy using paired sample t-test (P < 0.001). The mean TCB in neonates weighing 2500 to 3000 g after phototherapy was 8.57 ± 2.16 mg/dL and the mean TSB was 8.35 ± 2.13 mg/dL. No significant difference was observed between the TCB and the TSB after phototherapy in neonates weighing 2500 to 3000 g (P = 0.6).
Abbreviations: TCB, transcutaneous bilirubin; TSB, total serum bilirubin.
aValues are expressed as mean ± SD.
bt-test: Dependent sample t-test.
cMean differences are significant at the 0.05 level.
The mean TCB in neonates weighing 3001 - 4000 g before phototherapy was 10.23 ± 2.5 mg/dL and the mean TSB was 8.7 ± 2.74 mg/dL. The results of the paired sample t-test revealed that the means the TCB and the TSB were significantly different before phototherapy (P < 0.001).
The mean TCB in the neonates weighing 3001 - 4000 g after phototherapy was 7.99 ± 2.5 mg/dL and the mean TSB after phototherapy was 7.91 ± 3.43 mg/dL. The results of the paired t-test revealed no significant difference between the TCB and the TSB after phototherapy in neonates weighing 3001 - 4000 g (P = 0.88).
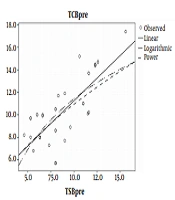
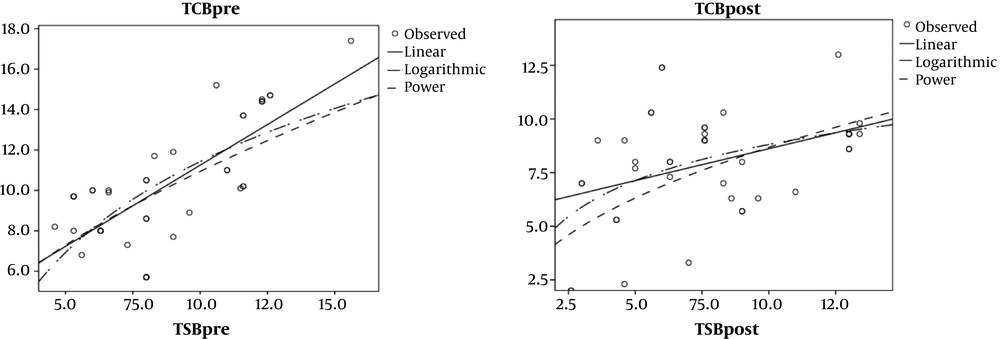
The results of the Pearson correlation test (Table 2 and Figure 1) showed a correlation between the TCB and the TSB in neonates weighing 2500 to 3000g before phototherapy (r = 0.58, P < 0.001). Although a low correlation between the TCB and the TSB was obtained in these neonates after phototherapy, it was still statistically significant (r = 0.33, P < 0.05).
aCorrelation is significant at the 0.05 level.
In addition, the results of the Pearson correlation test revealed a strong correlation between the TCB and the TSB in neonates weighing 3001-4000g before phototherapy (r = 0.74, P < 0.001). However, measurements showed a low correlation between the TCB and the TSB in these neonates after phototherapy (r = 0.40, P > 0.05).
In the present study, sensitivity and specificity of TCB measurements were investigated with respect to TCB levels using the ROC curve. The highest sensitivity and specificity was related to bilirubin at the levels between 6 - 8 mg/dL (cut off value), thus, the sensitivity was one (100%) and specificity was 90% for bilirubin at the levels of 6.7 mg/dL (Table 3). Therefore, this indicates acceptance of reliability of the BiliChek in determining the TCB. In addition, the PPV and NPV in the TCB relative to the TCB cut-off 6 values were 29 and 56, respectively. In other levels of bilirubin, the sensitivity and specificity were different. In the higher sensitivity, the specificity was lower, and vice versa.
| TCB Measurement, mg/dL | Sensitivity, % | Specificity, % |
|---|---|---|
| TCB < 8 | 100 | 90 |
| TCB 8 - 12 | 69 | 80 |
| TCB > 12 | 20 | 89 |
Abbreviation: TCB, transcutaneous bilirubin.
5. Discussion
Our study was implemented to compare the accuracy of TCB versus TSB before and after phototherapy and determination of the sensitivity of TCB.
Based on the results, we can say that the transcutaneous measurement of bilirubin can be a good screening device alternative for the TSB before phototherapy. Also, TCB measurement by special devices had been documented as an effective screening test for predicting hyperbilirubinemia before phototherapy.
However, it is seen that TCB measurements could not provide a reliable estimation after phototherapy. Phototherapy is likely to reduce correlation between TSB and TCB. Phototherapy causes photo isomerization of cutaneous bilirubin, which leads to the elimination of bilirubin without the need for conjugation. Therefore, cutaneous bilirubin concentration reduces after phototherapy, which will reduce the correlation between TCB and TSB after phototherapy. On the other hand, it seems that TCB is very reliable in neonates who are under phototherapy for 12 hours (not any longer). In our study, the duration of the implementation of TCB after phototherapy was 24 hours, which can reduce the reliability of this device.
Also, results of ROC showed that in the bilirubin values above 8 mg/dL, the specificity of the device and NPV increase, and sensitivity decreases. However, in bilirubin values below 8 mg/dL, sensitivity of the device and PPV increase and specificity decreases.
Our study indicated that age, weight, birth weight, and gestational age could not effect the correlation between the TSB and TCB. The results of Luca et al. and Babani et al.’s studies indicated that birth weight and preterm or term can’t affect the correlation between the TSB and TCB (19, 20). However, the results of some studies showed that skin color and gestational age are factors reducing the accuracy of the TCB (17, 19).
De Luca and Dell’Orto claimed that TCB would not affect preterm neonates due to variables influencing the bilirubin passage from circulation to the skin (PH, body temperature) (21). Furthermore, types of delivery probably change the results of TCB. Afjeh et al. considered that neonates who delivered by cesarean might stay longer in the nursery, thus, this was a more probable hyperbilirubinemia. This might have an effect on the results of TCB (22).
The findings of this study were consistent with the research of Radfar et al. (1). They found that TCB had a positive correlation with TSB before phototherapy. However, this correlation was significantly lower after phototherapy (1).
Results of the study by Pendse et al. showed that TCB had a positive correlation with TSB before phototherapy (r = 0.903, P < 0.001). In regards, TCB had an over positive correlation after the initiation phototherapy (r = 0.918, P < 0.001) (14). However, in our study, TCB had a positive correlation between the TCB and the TSB prior to initiation of phototherapy, however, there was a low correlation between the TCB and the TSB after phototherapy.
In Mansouri et al.’s study, the high correlation coefficients (r = 0.89) were found between TCB and TSB. In their study, the sensitivity and specificity of bilirubin levels was obtained between 12 - 15 mg/dL; PPV and NPV were 69.2 and 93.9 for TCB, respectively. In their study, the sensitivity decreased and the specificity increased at the bilirubin levels less than 12 mg/dL (18). In the current study, the specificity increased and the sensitivity decreased by reducing the levels of bilirubin, and vice versa.
In the study of Sajjadian et al. the highest level of sensitivity for the TCB was reported in the mean of TCB > 10 (sensitivity = 100%, specificity = 86%) and the NPV levels were variables from 0.6 to 1.0 and 0.29 to 0.81. In the present study, the highest sensitivity and specificity were related to bilirubin levels of 6.7 mg/dL (sensitivity = 100%, specificity = 90%) (23).
Kurokawa et al. stated that the reliability of the TCB method in the early days to seven days after birth is less than the following days, which is because the skin at these times doesn’t have enough growth. In their view, TCB is important from the eighth day after birth (15).
Babani et al. believed long-term phototherapy reduces the correlation between the TSB and the TCB, due to the fact that phototherapy changes the physical properties of the skin and the light isomerization. Therefore, this leads to the excretion of the bilirubin without conjugation, thus, TCB measurements will be lower after phototherapy (20).
Afanetti et al. claimed that the TCB technique could be used before phototherapy (17).
In the current study, transcutaneous bilirubin was measured in the neonates. If the study implemented on preterm neonates, another result would probably be achieved. Therefore, it is recommended that further studies be conducted on preterm neonates, and more studies with larger samples and a longer time can be conduct to achieve the reliability of the results. Due to the changes in the TSB and the TCB, it may be relating to the starting time of phototherapy, age, sex, and climatic conditions; therefore, it is suggested that further studies should be performed on this basis. It is possible that the use of different devices could affect the results; therefore, it is better to compare them.
This study has major implications for the neonatal nurses and other health care workers. Due to the invasive nature of the TSB procedure, the TCB method can be used to reduce the necessity for blood sampling in neonates. Therefore, results of this study would be used for developing countries where the rate of hyperbilirubinemia is high and there is an unavailability of micro-methods for bilirubin estimation in laboratories.
5.1. Limitations
Limitations of this study were the lack of control for interferer variables (age, sex, culture, etc.). In addition, due to the fact that the neonates were treated by phototherapy at different times and were out of control of the researchers, the different findings would most likely have resulted.
5.2. Conclusions
According to the results of the present study, we can claim that TCB procedure can be a viable alternative to the TSB technique, especially in the initiation of phototherapy with bilirubin levels of 6 - 8 mg/dL and in term of neonates. However, it is not a device with high accuracy after phototherapy.
Considering the fact that the TCB procedure be introduced as a non-invasive nature and is a no pain procedure, which prepares quick results within a few seconds, the BiliChek device can be used as a useful screening tool in term of neonates with hyperbilirubinemia.
It is recommended to the physicians and nurses that the TCB procedure is better for controlling and diagnosing hyperbilirubinemia, however, the TSB must be carried out in relation to the decision for phototherapy or exchange transfusion.