1. Introduction
Cutis laxa (CL) is a rare heterogeneous disorder of connective tissue characterized by loose, hypoelastic, wrinkled, sagging and redundant skin resulting in a prematurely aged appearance. It can be inherited or acquired, but inherited cases are more common. Inherited CL has considerably heterogeneous etiology and may be presented as autosomal dominant, autosomal recessive and X-linked recessive (1, 2). The autosomal recessive form of CL has most commonly been reported (3, 4). CL is separated into subtypes based on the genetic basis of the disorder. Autosomal recessive cutis laxa type 2B (ARCL2B; OMIM 612940) is caused by homozygous or compound heterozygous mutation in the pyrroline-5-carboxylate reductase 1 (PYCR1) gene, which is located on 17q25.3 (5, 6). Typical features of ARCL2B are wrinkled loose skin, progeroid appearance, developmental delay, growth retardation, and joint laxity (7). Patients may also have distinctive dysmorphic facial features including triangular face, high forehead, large ears, prognathism, hypotelorisms, bulbous nose, epicanthal folds, and blue sclera. Affected individuals may also suffer from skeletal anomalies, hypotonia, and variable central nervous system involvement (6, 8, 9). Using whole exome sequencing, we identified a homozygous mutation, c.722C>A, in PYCR1 gene, and a subsequent diagnosis of ARCL2B was made.
2. Case Presentation
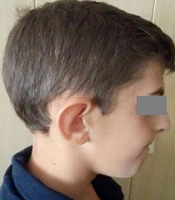
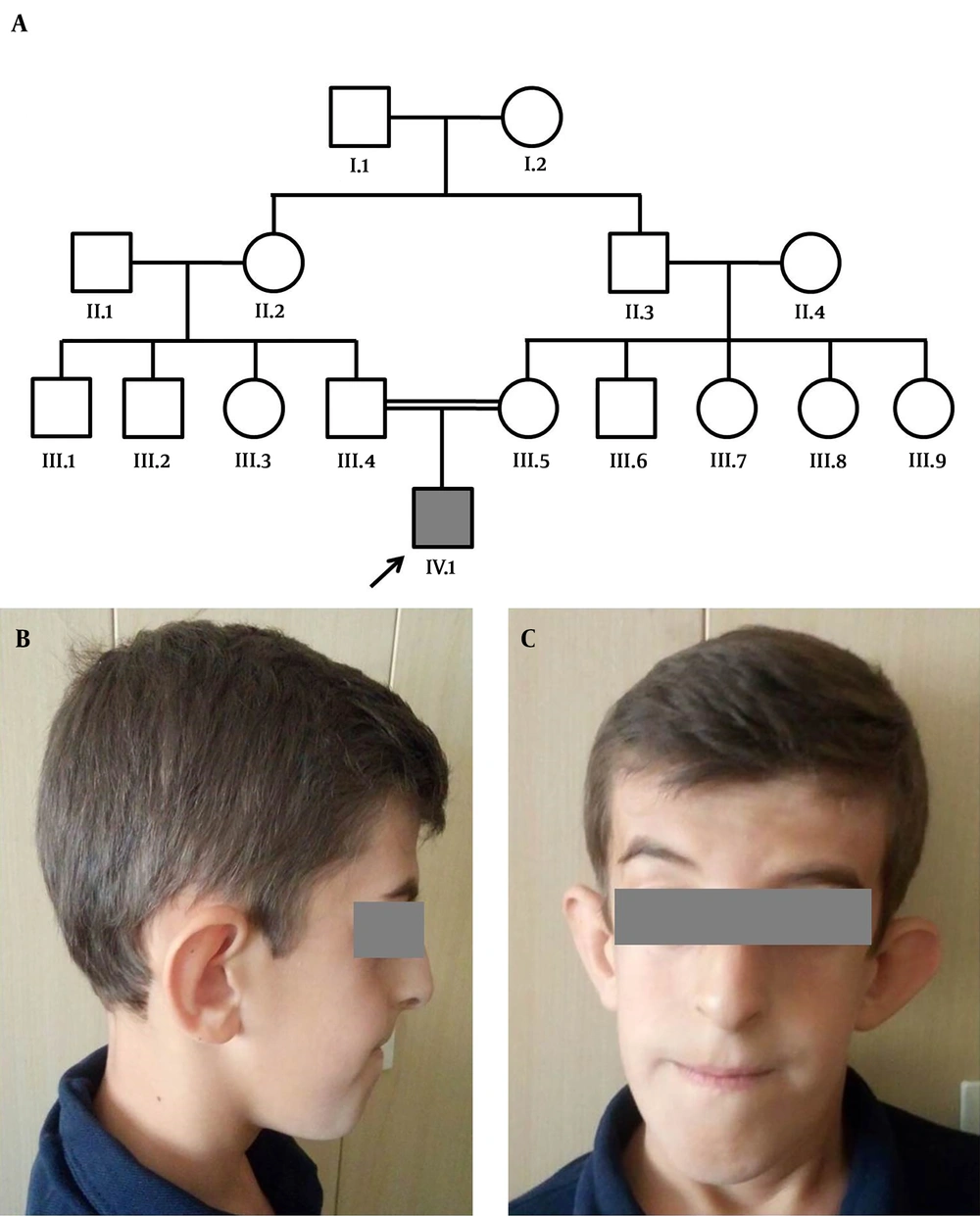
We reported a case on a 9-year-old boy (Figure 1) referred to our genetic counseling center due to his intellectual disability, developmental delay, and dysmorphic appearance. He was born as the first child of his consanguineous Iranian parents at 36 weeks of gestation via cesarean section. His birth weight was 2080 g (< 5th centile), length was 49 cm (25th centile), and head circumference was 30 cm (< 5th centile). No remarkable family history was reported. He could sit independently at 9 months, and was able to walk at the age of 32 months. On examination at the age of 9 years, his weight was 29.9 kg (< 5th centile), length 141 cm (90th centile), and OFC was 49.5 cm (50th centile).
A, Pedigree of the family presenting autosomal recessive cutis laxa type 2B. Squares and circles represent males and females, respectively. An arrow indicates the proband. Filled symbol refers to the patient (IV.1) and clear symbols represent normal individuals. The parents are consanguineous (III.4, III.5). B and C, Facial characteristics of the patient. Note the prematurely-aged appearance, triangular-shaped face, prognathism, large ears, and bulbous nose.
Clinical findings were lax wrinkled skin, intrauterine growth retardation, joint hyperlaxity, hypotonia, facial dysmorphisms including triangular-shaped face with a prematurely-aged appearance, prognathism, large ears, bulbous nose, broad nasal bridge, down-slanting palpebral fissures, and strabismus (Figure 1B and C). The patient also suffered from intellectual disability and developmental delay. He had a learning disorder as well as a language delay. His learning difficulties required special education.
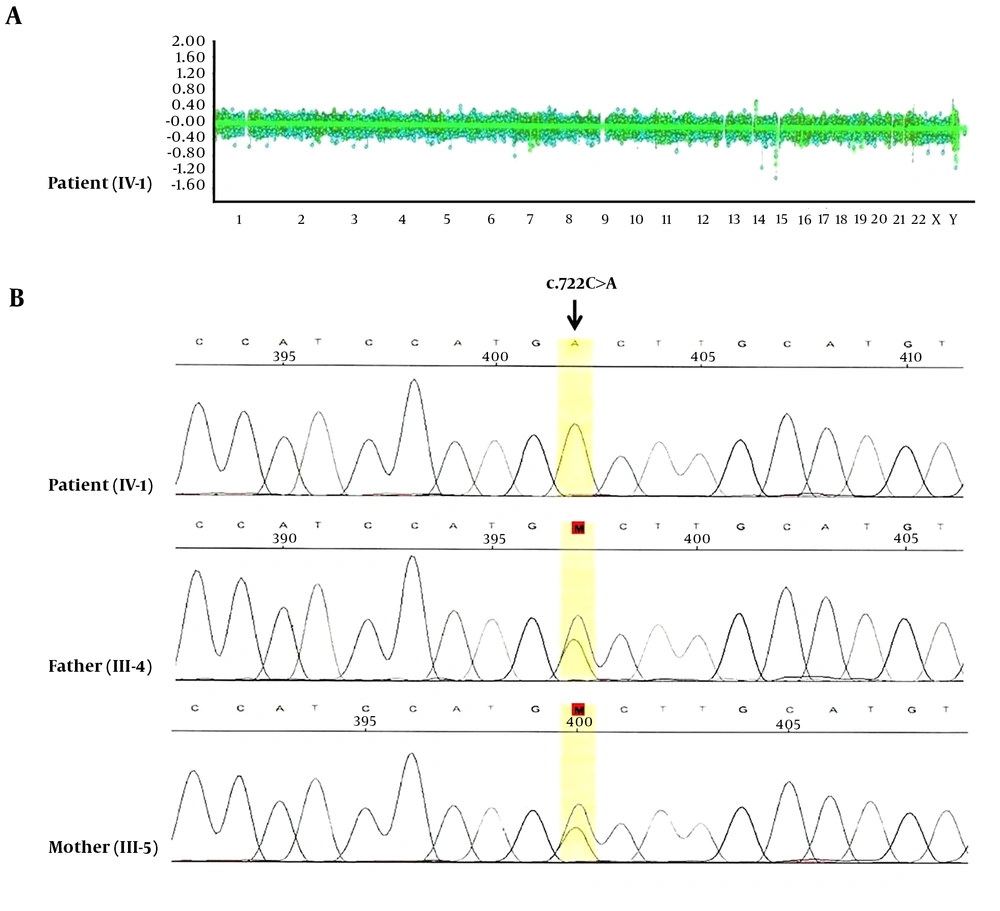
Conventional cytogenetic studies gave normal results. In addition, no pathogenic copy number variation (CNV), neither gain nor loss, was identified by array-CGH (Figure 2A). Whole exome sequencing (WES) was performed on the genomic DNA of the patient using Illumina Hiseq 4000. We found a homozygous missense mutation (c.722C>A; p.Ala241Asp) in exon six of the PYCR1 gene (NM-006907), which was confirmed by Sanger sequencing (Figure 2B). Both parents were heterozygous carriers of the causative genetic variant. The patient’s clinical phenotype, along with the PYCR1 mutation, was consistent with ARCL2B.
3. Discussion
We identified a homozygous PYCR1 missense variant (c.722C>A) in an Iranian boy with intellectual disability, developmental delay, cutis laxa, and dysmorphic facial features. The mutation leads to a change in the amino acid at position 241 of the protein from alanine to aspartate. Mutations in the PYCR1 gene can cause ARCL2B. Dimopoulou et al. reported this variant as a pathogenic variant in two Turkish patients (10). Another variant at the same position, c.722C>T; p.Ala241Val, has also been described to be associated with ARCL2B in a Brazilian patient (11).
We compared the clinical findings of our patient with previously reported patients with the same mutation (Table 1). Overall, the facial features and skin findings were similar. The patient presented here shared some clinical features with previously reported patients, such as mental retardation, developmental delay, psychomotor retardation, joint hyper laxity, and hypotonia. Although previous cases had osteopenia and suffered from microcephaly, hip dislocation, and adducted thumb, our patient lacked such phenotypes. In contrast, strabismus of the current patient was not found in the previous case.
| Author/Reference | Dimopoulou et al. (10) | Dimopoulou et al. (10) | Dimopoulou et al. (10) | Scherer et al. (11) | Our patient |
|---|---|---|---|---|---|
| Case number | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 |
| Origin | Turkey | Turkey | France | Brazil | Iran |
| Gender | NK | NK | NK | Male | Male |
| Mutations | |||||
| Status | hom | hom | het, het | hom | hom |
| cDNA | c.722C>A | c.722C>A | c.722C>T, c.138 + 2T>C | c.722C>T | c.722C>A |
| Consequence | p.Ala241Asp | p.Ala241Asp | p.Ala241Val, Splicing | p.Ala241Val | p.Ala241Asp |
| Exon | 6 | 6 | 6, 2 | 6 | 6 |
| Signs and symptoms | |||||
| Lax wrinkled skin | NK | + | + | + | + |
| IUGR | NK | + | + | NK | + |
| Hypotonia | + | + | + | - | + |
| Psychomotor retardation | + | + | + | + | + |
| Dysmorphic features | + | + | + | + | + |
| Triangular face | + | + | + | NK | + |
| Large ears | + | + | + | NK | + |
| Prominent chin | + | + | + | + | + |
| Postnatal growth delay | NK | - | - | + | - |
| Microcephaly | NK | + | - | - | - |
| Joint hyperlaxity | NK | + | + | + | + |
| Thin, translucent skin | NK | + | - | NK | + |
| Hip dislocation | NK | + | - | + | - |
| Hernias | NK | - | + | + | - |
| Cataract/corneal clouding | NK | - | - | - | - |
| Strabismus | NK | - | + | NK | + |
| Blue sclerae | + | + | - | NK | - |
| Adducted thumb | NK | - | - | + | - |
| Osteopenia | NK | + | NK | + | - |
| Wormian bones | NK | + | NK | NK | - |
| Late fontanel closure | NK | + | + | + | - |
| Corpus callosum dysgenesis | NK | - | NK | - | - |
| Athetoid movements | NK | - | + | - | - |
Abbreviations: het, heterozygous; hom, homozygous; IUGR, intrauterine growth retardation; NK, not known.
Missense mutations compose the main part of mutations causing this disease. The majority of the missense mutations were located within exons four to six, which encode the most highly conserved parts of the PYCR1 protein containing many residues involved in enzymatic function (12). PYCR1 encodes pyrroline-5-carboxylate reductase 1, a housekeeping mitochondrial enzyme, that catalyzes the last step in proline biosynthesis through NAD(P)H-dependent reduction of pyrroline-5-carboxylate (P5C) to L-proline (13).
In conclusion, this study identified a homozygous missense variant (c.722C>A; p.Ala241Asp) in a patient from a consanguineous Iranian family. PYCR1 mutations at the homozygous state results in ARCL2B. Our findings confirmed the previous two reports for ARCL2B patients with similar mutation. These results expand our insight for the clinical phenotype of ARCL2B. Further reports are required to better understand the PYCR1-related phenotypes, genotype-phenotype association, pathophysiology, and epidemiology to reach a critical number of patients for prevention or therapeutic studies.