1. Background
The celiac disease commonly involves the gastrointestinal tract. It is caused by immunological pathways. Gluten and its related prolamins are the causes of immunological process and disease onset (1). Although celiac disease is primarily a digestive tract disease, it can cause complications in most organs of the body (2). Epidemiologic studies report a global increase in the prevalence of the celiac disease, with different distribution patterns. For example, in a study in Europe, its prevalence was high in Finland (3), but lower in Germany and Italy (0.3% and 0.7%, respectively) (4).
Serologic screening studies report the increased incidence of celiac disease by two to five times over the past 50 years (5). Although celiac disease is currently diagnosed based on duodenal sampling and pathologic reports, the first step in the initial examination necessary to deal with suspected cases of celiac disease is always serologic tests (6). Common serologic tests to diagnose celiac disease include anti-tissue transglutaminase (tTG) and anti-endomysial antibody (EMA). A more recent test is anti-deamidated gliadin peptide, which is not as common as the first two tests, but it has high sensitivity and specificity and is reliable. Anti-gliadin antibodies are not commonly used (7) due to their low sensitivity and specificity.
Usually, the test of choice for celiac disease is tTG. In suspected cases of IgA deficiency, it should simultaneously be requested with its serum level (1). This early assessment method is also suggested in most reputable references (2). The employment of different tests depends on the geographical region, accessibility, and the need for a more accurate diagnosis of the disease. However, the results of various tests may be contrary to each other and we cannot always trust the results of a certain test in a single case; thus, sometimes a wider examination is required. The request for a test, however, depends on the clinical status, genetic characteristics, and other features of the patient (8).
In patients suspected of celiac disease with a proper serum level of IgA, a positive result of tTG and/or IgA-EMA is considered as a positive test; then, subsequent biopsy measures are taken (1). However, IgG tests should be requested in cases with IgA deficiency. IgA-based tests do not necessarily have to be positive in a patient with positive immunoglobulin G tests (1).
Some studies suggest that endoscopy is not required in children with tTG of above 100 for whom the symptoms of the disease improve following a gluten-free regimen (9). Therefore, the importance of tests and their roles in early diagnosis should be taken into account as there are different views on the possibility of changing the diagnostic criteria for celiac disease (10).
2. Objectives
The current study aimed at comparing the values of tTG and IgA/IgG-EMA tests in patients with definitive celiac disease to determine the compatibility of the tests, the test with the most positive results, and the test with the highest celiac-specific diagnostic value. The results of the study help clinicians to request a set of tests with higher sensitivity and specificity for the diagnosis of celiac disease.
3. Methods
This was an observational, descriptive, analytical study for the evaluation of diagnostic tests of celiac disease. The sample size was set to a minimum of 50, based on two studies by Baudon et al. and Vitoria et al. (11, 12). Patients referring to a Pediatric Gastroenterology and Liver Clinic, children who were referred from Golestan province to this clinic with celiac-related symptoms, and suspected cases, the initial and complete tests were performed for chief complains and celiac disease. In our center, the first step for the evaluation of celiac disease includes the complete panel test of IgG/IgA-tTG and IgG/IgA-EMA. We believe that the complete panel test is superior to only IgA-tTG because of more opportunity for screening positive cases. The celiac diagnostic algorithm at our center is applied for all suspected patients to diagnose the maximum number of disease cases; thus, no case is missed due to a negative test. If one test of this panel is positive, we perform endoscopy and biopsy for definitive diagnosis. The inclusion criteria were a positive result at least in one of the panel tests and a definite diagnosis of celiac disease based on pathology findings of biopsy (March 3). The duration of the study was from January 2016 to January 2017. The exclusion criteria were a negative test for celiac disease, a biopsy not compatible with celiac disease or march 0, 1, or 2, and parental disagreement for endoscopy. A demographic questionnaire was completed for each child and the collected data were analyzed with SPSS version 16 using statistical indices such as frequency and percentage; moreover, the kappa coefficient of the agreement was calculated.
4. Results
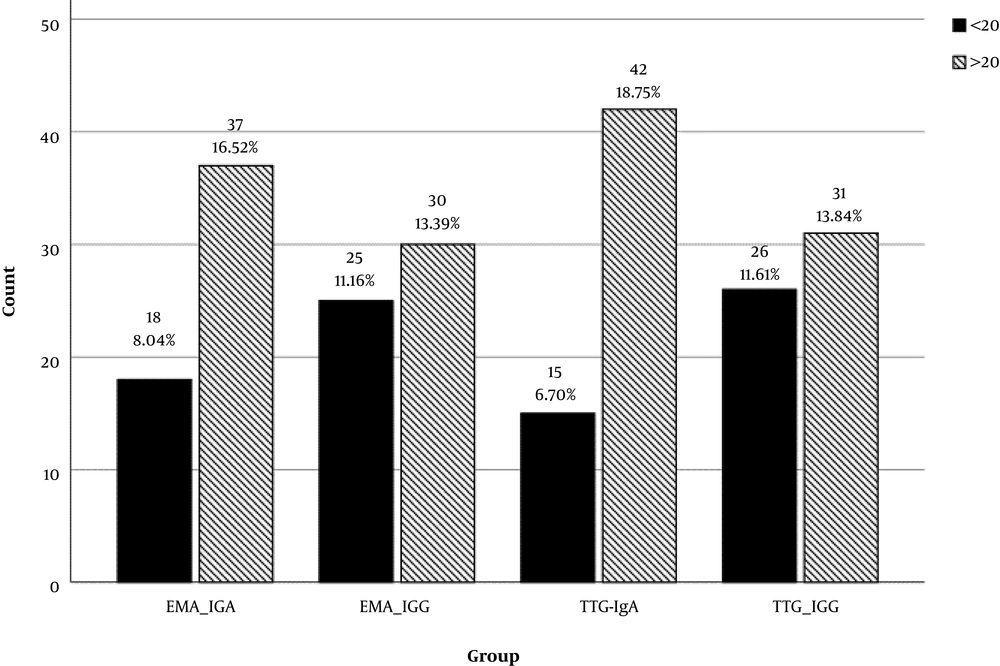
Of the 54 patients diagnosed with celiac disease, 29 were females and 25 were males (54% and 46%, respectively). The age range of the patients was one to 15 with a mean of 6.7 years. The quad screen test was performed for all patients and the IgA level was also measured. The positivity cut off was considered 20 for all the tests based on different laboratory criteria; the value of > 20 was considered positive and values equal or less than 20 was considered negative. Figure 1 shows the positive and negative rates of each of the quad screen tests. The highest positivity rate belonged to TTG-IgA, and the IgA-type tests, were more positive compared with IgG tests.
More than one test was positive in 81.5% of the patients; there were some cases with two and three positive tests. All four tests were positive in 16 patients. Only one test was positive in 18.5%, with the highest incidence belonging to TTG-IgA in four patients (Table 1).
| Test Positivity | Frequency | Percentage | Valid Percentage | Cumulative Percentage | |
|---|---|---|---|---|---|
| Valid | More than one is positive | 44 | 81.5 | 81.5 | 81.5 |
| TTG-IGA | 4 | 7.4 | 7.4 | 88.9 | |
| TTG-IGG | 2 | 3.7 | 3.7 | 92.6 | |
| EMA-IGA | 3 | 5.6 | 5.6 | 98.1 | |
| EMA-IGG | 1 | 1.9 | 1.9 | 100.0 | |
| Total | 54 | 100.0 | 100.0 |
Based on the literature, TTG-IgA is considered the reference test; thus, the agreement coefficients of other tests were calculated with TTG-IgA. The TTG-IgA test was positive in 39 out of 54 patients. In these patients, 31 cases had IgA-EMA-positive results and the rest had negative results. In 15 cases, the reference test was negative; however, in this group, five patients were positive for IgA-EMA and the rest of them were negative. The agreement coefficient between IgA-EMA and TTG-IgA tests was 0.435, which was statistically significant (Table 2). The agreement coefficient between the IgG-EMA and TTG-IgG tests was 0.026, which was very low without statistical significance.
| EMA IgA | Total | |||
|---|---|---|---|---|
| < 20 | > 20 | |||
| TTG IGA | ||||
| < 20 | ||||
| Count | 10 | 5 | 15 | |
| % within TTG IGA | 66.7 | 33.3 | 100.0 | |
| > 20 | ||||
| Count | 8 | 31 | 39 | |
| % within TTG IGA | 20.5 | 79.5 | 100.0 | |
| Total | ||||
| Count | 18 | 36 | 54 | |
| % within TTG IGA | 33.3 | 66.7 | 100.0 | |
| Symmetric Measures | Value | Asymptotic Standard Errora | Approximate Tb | Approximate Significance |
| Measure of agreement (kappa) | 0.435 | 0.131 | 3.223 | 0.001 |
| N of valid cases | 54 | |||
aNot assuming the null hypothesis.
bUsing the asymptotic standard error assuming the null hypothesis.
The patients were also evaluated for the reasons for referring to the center (Table 3). The most common causes of referring to the center related to celiac disease were growth failure as weight loss in 21 patients, followed by abdominal pain.
| Chief Complain | Frequency (%) |
|---|---|
| FTT | 21 (31.3) |
| Abdominal pain | 14 (20.9) |
| IDDM screening for celiac disease | 2 (3.0) |
| Short stature | 5 (7.5) |
| Chronic diarrhea | 5 (7.5) |
| Other complaints | 9 (13.4) |
| Follow-up for celiac disease | 11 (16.4) |
| Total | 67 (100.0) |
5. Discussion
A reliable test has more than 95% compliance with the standard criteria. The optimal threshold, the cutoff point, or the maximum upper limit of normal (ULN) should also be determined (1).
Celiac disease is characterized by highly specific autoantibodies against its common antigen, tissue transglutaminase (TG2). The current model for the diagnosis of celiac disease includes clinical suspicion, positive laboratory tests, duodenal sampling, and auxiliary findings of HLA DQ2/DQ8; indeed, the latter test is not performed sometimes (13). A small bowel biopsy, with the pathology report of villous atrophy, increased intraepithelial lymphocytes, crypt hyperplasia, and a gluten-containing diet is the diagnostic criterion or gold standard for the celiac disease diagnosis (14). Considering the outcomes and the high costs of endoscopy and biopsy, as well as the high prevalence of the celiac disease, there is a strong tendency toward replacing less invasive tests for diagnosis. Since the sensitivity and specificity of the serologic tests are almost optimal, it is increasingly being questioned that perhaps these tests alone may be sufficient to confirm the diagnosis; therefore, in some cases, a biopsy of the intestine can be avoided (9).
In our study, TTG-IgA had the highest positivity rate; according to various papers and guidelines, it is accepted as a valid test in the first step if it is provided by total IgA; in the case of lower levels of IgA, which are more common in celiac disease, IgG tests should be preferred to. However, positive TTG-IgA is not reported in all cases. Some studies indicated that despite the normal levels of IgA, the test was negative in a large number of patients; however, 15 cases in the current study had such conditions, which is highly remarkable.
The testing resulted in false-negative results in 15 cases. According to Table 1, in 10 cases, only one of the tests was positive that was a test other than TTG-IgA in six cases, indicating that even the various tests may be positive if tested alone; thus, we cannot always rely on the result of one test. Different studies used various tests to diagnose celiac disease, which depends on their availability and regional characteristics. Using a certain test makes it possible that a number of patients remain undiagnosed (15). For example, different assessment methods are available for TG2 that due to the lack of a particular international comparative standard, the results cannot be evaluated in a certain amount of immunoglobulin (1). Positive, negative, and interstitial values are differently interpreted in different kits that affect their specificity and sensitivity (15).
In a meta-analysis by Giersiepen et al., both tTG- and EMA-IgA tests had a high diagnostic value for celiac disease, but anti-deamidated gliadin peptide was more effective in tracing celiac disease; common anti-gliadin tests also had low accuracy (6). An important strength of our study was that the agreement coefficient was determined. There is a logical agreement between different tests; IgA-based tests had higher positive coefficients and higher agreement coefficients with other references, but the IgG-based tests had lower agreement coefficients. The agreement coefficient between the tests indicates that the same diagnostic value should be considered for all the above-mentioned tests. If the agreement coefficient is the unity for two different tests, one of them can be excluded. But, similar results were not obtained in the current study; in general, one of the celiac disease tests cannot be completely excluded due to compliance and agreement. This, of course, is apart from the exclusion of anti-gliadin tests, which, due to their low sensitivity and specificity, are not much used.
In the Baudin study, 28 out of 30 children were positive for tTG-ab, but the two remaining cases were negative, including a patient with IgA deficiency and an infant. Of them, 27 were positive for EMA and no significant difference was observed between the two tests (11). Vitoria et al. found similar results in a study on 26 patients (12). Our study was conducted on a two times larger sample and certainly higher negative cases can be attributed to this difference; in populations with a higher frequency of celiac disease, this number even increases.
Wolf et al. in a study evaluated the diagnostic value of various celiac tests. Based on their findings, IgA-tTG had a diagnostic value without the need for a biopsy when it was 10 times higher than the normal range; if it was < 1, celiac disease was ruled out, but there was no consensus on about 4% of the pathologic criteria among the pathologists (16). The discrepancy between the test results in the current study also confirmed the application of various tests to all patients.
Werkstetter et al. acknowledged the above-mentioned study and believed that a biopsy can be neglected in some special cases (17). In their study, it was emphasized that children could be diagnosed with celiac disease without endoscopy. Diagnosis based on a 10 times increase in tTG level plus positive EMA test and at least one celiac-related complain can reduce the risk of endoscopy and anesthesia in about 50% of children. Their study did not consider the HLA test as necessary for diagnosis (17).
Ermarth et al. evaluated the diagnostic value of serology, biopsy, tTG, and anti-deamidated gliadin peptide for celiac disease; they concluded higher diagnostic value for IgA-tTG than other tests and its positive predictive value was above 90% (18).
In another study, anti-gliadin and endomysial tests were evaluated; the results indicated 100% diagnostic sensitivity for IgG anti-gliadin and IgA-EMA and requesting IgA-anti-gliadin even increased the sensitivity. Although different tests were used in their study compared to our study, their conclusion was consistent with the current study findings (increasing the specificity of the evaluation by the addition of tests). However, as mentioned, the use of anti-gliadin tests is not recommended.
In a study by Ascher et al., on 55 children, it was concluded that the highest sensitivity and specificity could be obtained in children under five-years-old for anti-gliadin and in children above five-years-old and adults for EMA but only IgA-type tests had be employed (19).
The EMA specificity is approximately 98% to 100%. In some specialty labs, this test is considered as the standard reference of the celiac-specific antibody for the diagnosis of celiac disease (20). The sensitivity of anti-TG2 antibodies measured by ELISA is less than that of EMA and is highly dependent on the type of kits (6).
The protocol suggested for celiac diagnosis can vary based on costs, sensitivity, and specificity of the tool, as well as laboratory limits. In all studies, the cutoff point of the test was variable and could change the rate of false-positive or false-negative results. The view that clinicians should use only one test may be an economical and quick solution to solve problems, but in some areas, it is necessary to simultaneously use several tests to diagnose the disease as soon as possible. Celiac disease is one of the causes of growth retardation and has remarkable consequences in a patient’s life; some of its problems, such as short height, can be permanent and incurable if the treatment is delayed. Of course, protocols are slightly different in different regions (10).
5.1. Conclusions
Based on our study, it is suggested that both IgA and IgG types of tTG and EMA tests should be performed if available. Based on the current study, the suggested protocol increases the diagnostic sensitivity of celiac disease; if it is followed by endoscopy and biopsy, the diagnostic specificity of the evaluation can also increase. If the above-mentioned tests were not available, IgA tests should preferably be used.