1. Background
Despite the improvements in understanding of etiology and pathogenesis of infant anomalies, the congenital anomalies are the leading cause of death in 22% of infants’ anomalies. Health care for such infant anomalies impose a great cost on the government and families (1-3). The prevalence of major congenital anomalies varies among races and according to the exposure to some environmental factors (4). Consanguineous marriages play an important role in the development of congenital anomalies (4-7). Some studies cite that in the same region, the prevalence of anomalies in Muslims is more than the Christians. This is only related to religious beliefs about the Muslims’anti-abortion policies and consanguineous marriages (8).
2% - 3% of infants with anomalies have major structural anomalies at birth, which these are detected in 2% - 3% of them, by the end of the 5th year (1). However, most anomalies are not hereditary, and there is genetic disorder only in a slight part of those with birth defects, who seek for counseling. Also, about 10% of congenital anomalies are caused by teratogenic agents, including: chemicals, viruses (rubella, cytomegalovirus, and toxoplasmosis), environmental factors, physical factors (X-rays), and drugs. Infants whose mothers over consumed drug during pregnancy, were more likely to have congenital anomalies (2).
In addition, some congenital anomalies are associated with the baby sex, birth weight, and parental age, especially the age of mother and fetus (3). Consanguineous marriage increases the incidence of autistic autosomal recessive diseases in infants of these parents (4). The significant effect of some factors, such as the family history of genetic diseases, low birth weight, and premature infant, on congenital anomalies have been indicated in some studies, and the incidence of anomalies are ranging from 0.82% in Arak to 2.3% in India (9, 10).
According to the implemented studies, about 2% - 3% of infants have severe anomalies, a few of which are so problematic that may cause death, but in the rest of them disability and death can be prevented by early diagnosis and treatment (11). However, since it is possible to prevented many congenital anomalies, recognizing and preventing congenital anomalies in societies impose a far less cost compared to their treatment or rehabilitation (4).
2. Objectives
This study was conducted to determine the risk factors of congenital anomalies in infants in Akbar Abadi Hospital, Tehran, Iran, in order to provide a basis for genetic counseling and, if possible, prenatal diagnosis in subsequent children and creating a context for future studies.
3. Methods
This case-control study was conducted in Akbar Abadi hospital, Tehran, from April 2016 to
April 2017. To conduct this research, the data were collected from patients who referred to the maternity clinic of Akbar Abadi Hospital, from April 2016 to April 2017. In the current study, congenital anomalies of infants were reviewed based on pediatric examinations. In order to make the similarity between two groups, the control group was selected from the same hospital. The control group was selected from healthy infants at the same time, who were born in the hospital, and 83 healthy infants were randomly selected in each season to participate in the study.
Cases were selected within a year, from 10187 infants. Accordingly, 332 infants with confirmed congenital anomalies were considered as cases group, and they were compared to332 healthy infants as the control group.
The exclusion criteria included: (a) Parental dissatisfaction on participating in the study; (b) inaccessibility of parents. The maternal factors including age and diseases, consanguineous parents, father’s age, the history of abortion in mothers, residence place, mother’s smoking habit, the history of having a child with abnormality, and factors related to the baby, including weight, sex and Apgar score, along with other important points were recorded in the examination of the infants.
The data were collected using a questionnaire, which included infant and parental characteristics and insertion anomalies of the infants. A questionnaire was filled up for each infant, and a result was recorded in the questionnaire for the newly hospitalized infants after the baby was examined by a pediatrician.
The results for quantitative variables are expressed as mean and standard deviations (mean ± SD) and for the qualitative variables, they were expressed as count and percentages. The quantitative variables are compared using the independent t-test or Mann-Whitney U test. Moreover, the qualitative variables are compared using chi-square test or Fischer’s exact test. The unadjusted and adjusted odds ratios (OR) estimates of the congenital anomalies, were calculated using the simple and multiple logistic regression. Also, SPSS 19 was used for statistical analysis of the data. The significance level is considered to be less than 0.05.
4. Results
This study was conducted within a year. During this study, two groups of case and control were studied, which the case group included 332 infants with abnormality and control group included 332 healthy infants. Also, none of the infants in case group met the exclusion criteria, and all of them were included and analyzed in the current study. Unilateral Undiscerning Testis (UDT), hypospadias, and club-foot were the most common anomalies in premature and term neonates. The prevalence of anomalies were 13.8% for unilateral UDT, 13.5% for hypospadias, 13.5% for club-foot, 11.4% for bilateral UDT, 7.4% for low set ear, 6% congenital hydrocephalus, 3.9% microcephaly, 3/3% down syndrome, 3% cleft lip and cleft palate, and other anomalies were less prevalent. There have been multiple anomalies (at least 3) in 6% of infants. There were also 800 deficits, such as atrial septal defects (ASD), ventricular septal defects (VSD), and patent foramen ovale (PFO) based on echocardiography, and according to the need for cardiac counseling for infants during the first 3 days.
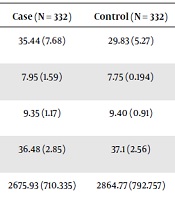
As Tables 1 and 2 shows, 60.8% of the infants of the case group and 62.3% of the control group were male. The gestational age of birth was 36.48 weeks in the case group and 37.1 weeks in the control group. Birth weight was 2675.93 gram in the case group and 2864.77 gram in the control group. The mean maternal age was 29.48 years in the case group and 27.9 years in the control group (Tables 1 and 2).
| Characteristics | Case (N = 332) | Control (N = 332) | P Valuea |
|---|---|---|---|
| Mean of father age (SD) | 35.44 (7.68) | 29.83 (5.27) | < 0.001 |
| Mean of apgar in one minutes (SD) | 7.95 (1.59) | 7.75 (0.194) | 0.059 |
| Mean of apgar in 5 minutes (SD) | 9.35 (1.17) | 9.40 (0.91) | 0.530 |
| Mean of gestational age (SD) | 36.48 (2.85) | 37.1 (2.56) | 0.003 |
| Mean of birth weight (SD) | 2675.93 (710.335) | 2864.77 (792.757) | 0.001 |
| Mean of maternal age (SD) | 29.48 (6.32) | 27.9 (5.89) | 0.001 |
Comparison of the Characteristics of the Cases (N = 332) and Control (N = 332) Groups
| Characteristics | Cases (N = 332) | Controls (N = 332) | Unadjusted Model (OR 95% CI) | Adjusted Modela (OR 95% CI) |
|---|---|---|---|---|
| Gender | 0.938 (0.686, 1.28) | - | ||
| Boy | 202 | 207 | ||
| Girl | 130 | 125 | ||
| Consanguinity of parents | 2.65 (1.51, 4.63) | 2.47 (1.25, 4.77) | ||
| Relative | 46 | 19 | ||
| Not relative | 286 | 313 | ||
| The type of delivery | 1.8 (1.31, 2.47) | 1.92 (1.31, 2.8) | ||
| C/S | 226 | 180 | ||
| NVD | 106 | 152 | ||
| Abortion | 2.544 (1.55, 4.15) | 3.02 (1.7, 5.27) | ||
| Positive | 59 | 26 | ||
| Negative | 273 | 306 | ||
| Maternal disease | 4.811 (3.11, 4.43) | 4.42 (2.7, 7.3) | ||
| Positive | 110 | 31 | ||
| Negative | 222 | 301 | ||
| Smoking | 2.409 (1.52, 3.81) | 2.47 (1.46, 4.2) | ||
| Yes | 66 | 31 | ||
| No | 266 | 301 | ||
| The history of child with abnormality | 3.295 (1.38, 7.82) | 3.27 (1.26, 8.41) | ||
| Yes | 22 | 7 | ||
| No | 310 | 325 | ||
| Mean of father age | 35.44 | 29.83 | 1.14 (1.11, 1.17) | 1.136 (1.1, 1.17) |
| Mean of birth weight | 2675.93 | 2864.77 | 1 (0.999, 1) | - |
| Mean of gestational age | 36.48 | 37.1 | 0.92 (0.87, 0.97) | 0.91(0.85, 0.97) |
| Mean of maternal age | 29.48 | 27.9 | 1.04 (1.017, 1.07) | 1.045 (1.02, 1.08) |
Association Between Congenital Anomalies and Potential Risk Factors Using Logistic Regression Analysis
About 17.8% of mothers in the case group and 7.8% of mothers in the control group have had abortion history in their previous deliveries. The rate of consanguineous parents was 13.9% in infants with abnormality and 5.7%in control group.
In the case group 68.1% of the infants were delivered through cesarean section and this rate was 54.2% in the control group, also 36.7% of mothers in case group and 41.3% of mothers in control group were experiencing their first gravid, 28.9% in the case group and 25.9% in the control group were second gravid, and 34.4% in the case group and 32.8% in the control group were experiencing their third and more.
In addition, most of the mothers in the control and case group were living in the city. The number of mothers with smoking habits were less in the control group compared to the case group, and also in both groups, the number of non-smoking mothers were higher than smoking mothers. The prevalence of these anomalies in case group was 28.9% in spring, 24.7% in summer, and 23.8% in autumn, and 22.6% in winter, and in control group, it was 25% in all seasons. Maternal diseases, such as diabetes, HTN, hypothyroidism, and etc. are observed in 33.1% of the case group and 9.3% of the control group.
Briefly, there was a relationship between consanguineous parents, maternal disease, the type of delivery, abortion history, mothers’ smoking habit, and the history of child with abnormality (P value < 0.05). There was not statistically significant difference between gender of infants, gravidity, place of residence, the season of birth, Apgar, Apgar score in 5 minutes, and gestational age, and they were almost the same (P value > 0.05).
Table 2 presents the effect of various potential risk factors on congenital anomalies using crude and adjusted OR (Table 2).
Based on the results, the OR adjusted estimates of congenital anomalies were 1.045 for maternal age (1.02, 1.08), 2.47 (1.25, 4.77) for consanguineous parents, 4.42 (2.7, 7.3) for positive maternal disease compared to negative maternal disease, 1.92 (1.31, 2.8) for C/S(cesarean section) compared to NVD (Natural Vaginal Delivery), 3.02 (1.7, 5.27)for positive abortion compared negative abortion, 1.136 (1.1, 1.17) for fathers’ age, 2.47 (1.46, 4.2) for smoking mothers compared to non-smoking mothers, 3.27 (1.26, 8.41) for mothers having children with abnormality compared to mothers not having children with abnormality, and 0.91 (0.85, 0.97) for gestational age.
5. Discussion
Major anomalies refer to disorders, which if are not corrected or cannot be modified in a timely manner, the normal functioning of the body is impaired or the lifetime of the individual is decreased. The cleft lip, cataract, hydrocephalus, and malignant cell are the major anomalies. In this study, the prevalence of anomalies in Akbar Abadi hospital was estimated 3.2% in one year. The total number of patients with congenital anomalies, reported in other studies, wasranged1.86%, and it was reported 3% - 5% in developed countries, approximately 4.3% in Taiwan, 7.92% for the United Arab Emirates, 2.46% for Oman, 2.7% for Bahrain, and 3.6% for India (12-16). The reported prevalence of congenital anomalies in Iran was 2.3% for Tehran, 1.01% for Gorgan, and 3.76% for Yazd (17-19). These results are partial estimations, since they have only been expressed based on the physical examination of the infants, and the additional anomalies, which are recognized by age or cause death in the fetus, are not considered. In the current study, only 6% of the anomalies were multiple. About two-thirds of congenital anomalies are isolated and located at one point of the body.
In this study, the potential risk factors for congenital anomalies were investigated. It was indicated that consanguineous, maternal disease, the type of delivery, abortion, smoking, fathers’ age, birth weight, the history of previous child with anomaly, and maternal age, were the strongest risk factors of congenital anomalies.
According to our findings, the prevalence of congenital anomalies was higher in boys compared to girls, but according to the logistic regression results, there was no significant relationship between the anomaly and gender. This finding was in agreement with other studies (20, 21). There was no relationship between the sex of infants with anomalies and year of birth. A study in Iran reported that the prevalence of anomalies in boys is higher than girls, and it was in agreement with our results (22). Another study (18) reported that infant boys were more affected than girls, but some studies reported that the prevalence of congenital anomalies is not affected by the gender of infant , therefore both genders had similar possibility of having congenital anomalies (19, 23, 24). Also in our results, the gestational age had an effect on anomaly, such that there was an increase in anomaly by decreasing gestational age. This result was in accordance with the results of a study conducted by Mohajer Shirvani et al. (25) in which there were a significant relationship between the age of pregnancy and the length of fetal kidney.
In scientific resources, consanguineous marriage has been mentioned as an important factor in the occurrence of congenital anomalies (26). According to our study, 13.9% of infants with congenital anomalies had consanguineous parents. In a study, 62.6% of parents of infants with congenital anomalies were related and 34.7% of them were not related, and the results of the current study are confirmed by these results (27). Genetic interactions in consanguineous marriage can lead to anomaly in infants. The prevalence of congenital anomalies in infants was higher in mothers with disease history, compared to the control group, and it means that the maternal disease can be a strong factor in anomalies. In a study conducted on Egyptian infants, maternal disease, especially diabetes, was 7.28% (28). This result can be due to the effect of the drugs consumed by the mother for the previous illness. Also, in mothers with a history of abortion in previous gravid, there was a significant relationship between anomalies of infants and the positive abortion. In a study, it was reported that there was history of abortion in 32.39%of mothers with congenital anomaly infants (28). This finding might be due to the secretion of hormones after abortion. The prevalence of anomalies were higher in infants with smoking mothers. Some studies reveal that there are positive correlations between maternal smoking and congenital anomalies (28, 29). There was a significant correlation between birth weight and the prevalence of anomalies, and there were also a relationship between the prevalence of anomalies, low birth weight, and prematurity. In a study by Rabah et al. it was reported that birth weight less than 2.5 kg was detected in 71.04% of patients with congenital anomalies. This finding can be due to the fact that infants with low birth weight are more likely to develop an anomaly. In this study, congenital anomalies ware more prevalent in infants who were born in the spring, and in the infants who were born in the winter, those had the least prevalent. According to previous studies, fertilization in autumn leads to an increase in the number of births, but the prevalence of congenital anomalies are not related to seasons (11, 20, 21, 30).This finding is because the pregnancy period was in seasons and mother was exposed to less sunlight and cold weather (31). Father’s age is another strong factor identified in the current study, such the infants with anomalies have aged fathers. In another study, it was reported in 29.99% of patients with congenital anomalies, fathers were above 50 year at the time of conception (28). This result can be due to low motility and low number of sperm.
As mentioned above, unilateral UDT, hypospadias, and club-foot were the most common anomalies. In some cases, other studies are in agreement with our findings and, in some cases, they are not. In a study in China, conducted by Ge Sun Zhe-Ming Xu in 2011, the 12-year prevalence of congenital anomalies were examined, and the results showed that, although there was an increased intestinal CHD intake, kidney failure, and hypospadias, the anorectal, poly-ductility, sin-ductility, and hydrocephalus were decreased (32). In a study in Nigeria, conducted by Ekwere et al. in 2011, the most common types of anomalies among 200 types, were respectively gastrointestinal anomalies (30.30%), the central nervous system anomalies (24.25%), and the others (13.5%) (33). There was an outbreak of congenital anomalies in Shari’ati Hospital, Tehran, in 2002 - 2004 by Hamideh Shajari. Among 3840 infants who were included in the study, 118 cases (1.3%) had anomalies. Anomalies were more prevalent in boys and the skeletal system and nervous system anomalies were the most common ones (34).
It was attempted to investigate as many patients as possible. However, we could not find more than 332 patients in a year. On the other hand, this study was performed only on infants who were born in Akbar Abadi Hospital from April 2016 to April 2017. Despite its limitations, this study could examine the effect of several potential risk factors on congenital anomalies.
5.1. Conclusions
The results of this study determined the potential risk factors for congenital anomalies in the considered population. It was concluded that maternal age, maternal disease, the type of delivery, history of abortion, father’s age, smoking, history of having child with abnormality, gestational age and consanguineous parents are the potential risk factors of congenital anomalies. In terms of adjusted logistic regression, maternal disease, the history of having child with abnormality, and the history of abortion were the most effective factors in anomalies. In the next step, the consanguineous parents, smoking, the type of delivery, and father’s age were important risk factors. Finally, maternal age and gestational age had significant effect on anomaly. There was no relationship between birth weight, gender and congenital anomalies. However, our study was an observational study, and other studies with larger sample size are needed to investigate exact effect of aforementioned factors.