1. Context
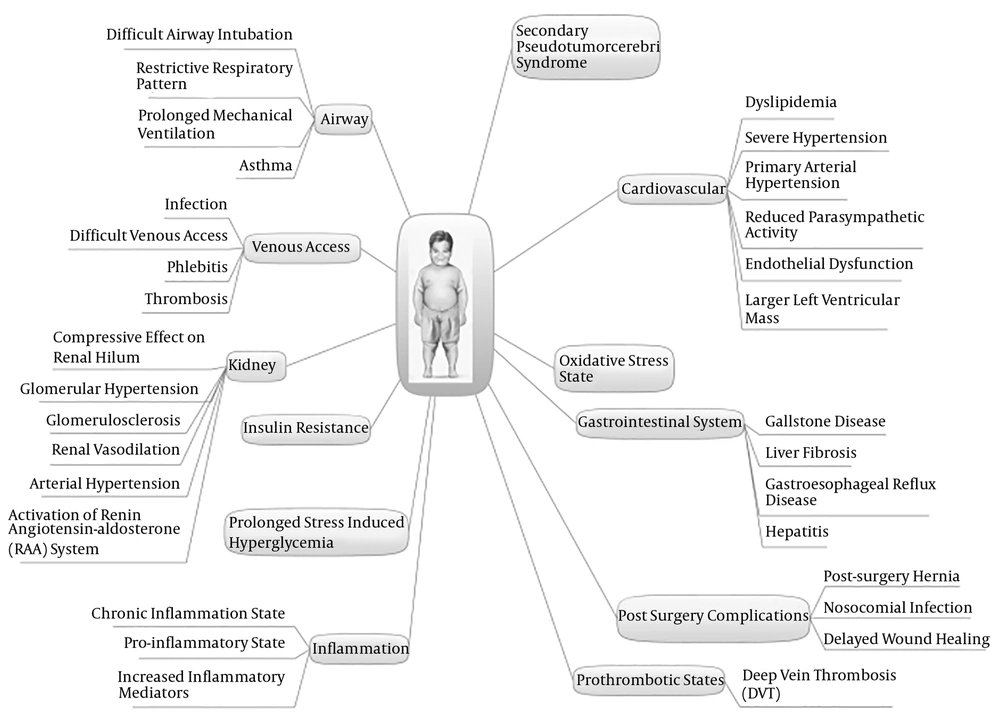
Childhood obesity is one of the serious health problems in the world. About 14% of Iranian boys and 10% of Iranian girls are suffering from general obesity (1). It is associated with comorbidities and complications including secondary pseudotumor cerebri syndrome, insulin resistance, hyperglycemia, dyslipidemia, increased risk of gastroesophageal reflux disease, hepatitis, liver fibrosis, gallstone, and antibiotic treatment failure (2-5). Endothelial dysfunction and reduced parasympathetic activity in obese children may result in primary arterial hypertension in critically ill patients, followed by larger left ventricular mass and severe hypertension in obese subjects in Pediatric Intensive Care Units (PICUs) (6, 7). Additionally, excess adipose tissue in the body, reduced lean mass and water levels may lead to changes in the volume of drug distribution throughout the body (8).
The airway management of obese patients in PICUs is challenging; for example, obesity may increase the risk of airway intervention requirement, prolonged mechanical ventilation, and obstructive sleep apnea (1, 9-11). Additionally, excess adipose tissue may lead to difficult peripheral and central venous access. Also, because of technical challenges and difficulties in alternative central venous access, catheters may be left longer, which increases the risk of catheter-related complications such as infections, thrombosis, and phlebitis (9). Obesity is an independent risk factor for surgical outcomes and surgical-related morbidities in children. For example, children with obesity are at an increased risk of post-surgery hernia, nosocomial infections, deep-vein thrombosis, and delayed wound healing (12).
Obesity-related diseases and complications and obesity-related challenges in common procedures in PICUs make it difficult to conduct the medical management of critically ill obese children and obese children are considered to be at a high risk of mortality and morbidity in PICUs (9, 13, 14). Obesity-related challenges in the management of obese children in PICUs are summarized in Figure 1.
In addition to the effects of inflammation and metabolic stress on metabolism nutrients, obesity is associated with alterations in the metabolic process of critically ill obese children due to the high risk of nutritional deprivation in PICUs (13-15). Therefore, it is critical to screen potential nutritional deficiencies and improve the optimal energy and nutrient delivery in obese patients in PICUs (14-16).
While under/overfeeding of critically ill obese children may lead to physiological instability and adverse clinical outcomes, routine behavioral, nutritional, pharmacological, and surgical interventions for obesity treatment are not yet assessed in critically ill PICU patients (17). Previous studies demonstrated inconsistent results in nutritional management strategies regarding calories and macro/micronutrient requirements in the medical management of children with critical illness and obesity. Therefore, the main objective of this study was to elaborate on the nutritional management approaches of critically ill obese children such as nutritional assessment, energy requirement, macro/micronutrient requirement, and additional supplementation intake.
2. Evidence Acquisition
This narrative review was conducted to provide updates on the nutritional management of critically ill obese children. The search was performed using a combination of MeSH terms and keywords on the National Library of Medicine’s PubMed, Scopus, Cochrane, and Embase as follows: “(((((“Child”[Mesh]) or “Pediatrics”[Mesh]) or “Infant”[Mesh]))) AND ((((((((((mortality) OR Obesity) OR Morbid obesity) OR morbidity) OR Complications) OR obesity Management)) AND (((((Intensive Care Units, Pediatrics) OR intensive care enteral nutrition) OR intensive care parenteral nutrition) OR critical illness) OR critical care))) AND (((Nutrition Assessment) OR Diet Therapy))))”.
Relevant publications were identified in English cited from 1st January 1990 to 31st December 2018. The reference lists of the relevant articles were also reviewed to ensure study identification. Any disagreements were discussed and resolved in the focus group panel by all four research investigators. Firstly, 329 articles were retrieved and 19 additional records were identified through other sources and all of them were screened for eligibility in title and abstract by four independent reviewers. Duplications were removed; then, 53 records were identified. The quality assessment of the studies was carried out using “the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials” and “the risk of bias in observational studies of exposures” tools in focus group panels (18, 19). Therefore, after the quality assessment for eligibility, 42 studies were included in this narrative review.
3. Results
3.1. Nutritional Assessment of Critically Ill Obese Children
Obese children admitted to PICUs constitute a nutritionally high-risk population (16). The initial nutritional assessment with a nutrition care program is necessary for all critically ill obese children, especially in complicated subjects (14, 16, 20).
The assessment of anthropometric parameters including mid-upper arm circumference (MAC), body weight, height/length, and BMI should be routinely performed to assess and follow the nutrition status of these children (21). Alterations in MAC and weight are correlated with cumulative energy and protein deficiency in PICU patients (22). In addition to the routine monitoring of weight, MAC, and other anthropometric parameters, nitrogen balance should be routinely measured during PICU stay and the diet should be modified in negative nitrogen balance cases (17). Moreover, as an indicator of visceral protein pool, the measurement of pre-albumin may be useful, but this test should be interpreted considering the inflammation phase and illness severity (17).
Obese children are at a higher risk of prolonged stress-induced hyperglycemia than normal subjects (13). Short-time stress could induce hyperglycemia in critically ill patients and remain beneficial since it provides glucose as an energy substrate in the hypermetabolism state to tissues; however, sustaining it in obese children may deteriorate the effect of oxidative stress by increasing free radicals and increased inflammatory cytokines, which may reduce immune system functioning (23).
Adipose tissue is not only an inert-fat storing tissue but it is also considered an endocrine organ secreting several hormones including leptin, resistin, visfatin, and adiponectin. These hormones play a major role in various body functions such as nutritional intake, control of sensitivity to insulin, and inflammatory process (24). These hormones can cause the activation of inflammatory pathways and significantly increase pro-inflammatory cytokine levels including tumor necrosis factor-α, interleukin-1b, and interleukin-6, as well as the chronic inflammatory state. Particularly, macrophage-related inflammatory activities may lead to insulin resistance and reduced immunity in obese subjects (25). Therefore, the inflammatory response of obese children to acute injury is differently exacerbated compared with non-obese children (13). Leptin, resistin, adiponectin, and inflammatory mediators secreted by adipose tissue, obesity-related dyslipidemia, and hyperglycemia are other factors involved in the progression of renal dysfunction in obese children admitted to ICUs (13). Chronic inflammation state and increased inflammatory mediators in obese children predispose them to pro-inflammatory and prothrombotic states and oxidative stress (20). Serum C reactive protein (CRP) can also be used as an indicator of inflammation in relation to pre-albumin. In post-surgical cases, a decrease in CRP levels in serum is associated with the elevation of pre-albumin levels, both of which indicate the anabolic phase of metabolism after acute injury (22). The derangements in fatty acid profiles can influence inflammation, organ function, disease process, and survival (26, 27). The derangements in fatty acid profiles may lead to inflammation, organ dysfunction, deterioration of disease process, and reduced survival (25). Finally, childhood obesity may lead to the development of nonalcoholic fatty liver disease (NAFLD) (28). On the other hand, the acute phase response is considered a risk factor for the development of fatty liver disease (29). The recommended routine nutritional assessment of critically ill obese children include lipid profile, glucose, phosphorus, liver function tests, and complete blood count (15, 30).
3.2. Energy and Macronutrient Requirement
Undernutrition in obese children may result in nutritional deprivation and leads to poor clinical outcomes, especially because of negative protein balance and muscle mass wasting (31, 32).
Overfeeding may result in electrolyte imbalance, respiratory, and cardiovascular complications and increase the length of ICU stay (17). Failure to recognize the hypermetabolic phase of metabolic stress response, reliance on standardized formulae/equations for energy expenditure, inaccurate weight measurements, confusion about which weight to use for obese patients (actual/ ideal body weight), and overestimating the degree of metabolic stress in the era of modern anesthesia and surgery may lead to the overfeeding of these patients in PICUs (10, 31). Optimal energy delivery to such obese patients is essential for sustaining the function of the immune, cardiovascular, and respiratory systems in the acute phase of the inflammatory response (32, 33). Insulin and growth hormone resistance following acute injury caused by increased serum counter-regulatory hormone concentrations could lead to catabolism (34). Optimal nutrition support provision is necessary to prevent the loss of lean body mass in critically ill children. Hypo-caloric feeding is also not recommended during ICU stay since it could increase catabolism in metabolic stress state (15, 17). On the other hand, the transient absence of growth, mechanical ventilation, sedation, activity reduction, and decreased insensible fluid loss during the acute phase of inflammatory response may lead to resting energy expenditure (REE) reduction. Obese children could have various REEs caused by differences in lean body mass, nature, and phases of the acute illness (35).
Table 1 represents cross-sectional/cohort clinical trials of energy expenditure estimation in children with obesity. It summarizes studies on the accuracy of predictive equations in comparison with measured REEs by indirect calorimetry (IC). As noted in Table 1, according to the studies, the most accurate REE predictive equations were SCHO-HTWT, Harris-Benedict, and Lazzer, in sequence (36-38). However, in the majority of other studies (80% of all relevant cross-sectional/cohort clinical trials), it is reported that none of the published equations (i.e., World Health Organization (WHO), Schofield and Harris-Benedict, etc.) was accurate (39-47). Notably, in a few studies, one or two equations were developed that were only accurate in the studied population group and had little generalizability (39-45, 47).
| Country | Authors’ Names | Number of Participants | Main Findings | Reference |
|---|---|---|---|---|
| Turkey | Acar-Tek et al. | 103 obese children and adolescents | All of the existing predictive equations were inaccurate in the estimation of REE for obese children.A new prediction equation was developed for local use. | (39) |
| Italy | Marra et al. | 264 obese adolescents | None of the previously published equations was accurate.The most suitable equations included the Lazzer equation in both males and females and Schmelz and Henry-1 equations in females. | (38) |
| Korea | Kim et al. | 52 obese children and adolescents | Harris-Benedict, Liu, Mifflin, and Molnar equations were the most accurate predictive equations (accuracy: 73%, 77%, 79%, and 87%, respectively). | (37) |
| China | Chan et al. | 100 obese children | None of the previously developed prediction equations was accurate.A new prediction equation was generated for local use. | (40) |
| Italy | Lazzer et al. | 574 obese children and adolescents | Two new equations were developed based on anthropometric parameters for obese Caucasian children and adolescents. | (42) |
| Germany | Schmelzle et al. | 82 obese children and adolescents | All of the published predictive equations were inaccurate and inclusion of lean body mass in REE prediction improved the accuracy. | (46) |
| France | Derumeaux-Burel et al. | 752 obese children and adolescents (n = 471 derivation and n = 211 validation) | New local accurate equations were established. | (41) |
| United States of America | McDuffie et al. | 502 obese children | None of the World Health Organization, Food and Agriculture Organization, United Nations University, Schofield, Molnar, Maffetis, and Tverskaya equations was accurate.Two new specific equations were developed. | (44) |
| USA | Tverskaya et al. | 110 obese children and adolescents | Previous equations did not accurately predict BMR.A new equation was generated. | (47) |
| USA | Kaplan et al. | 20 obese children | The SCHO-HTWT equation was the best predictive equation (95% +/- 17%). | (36) |
| Hungary | Molnar et al. | 136 obese children and adolescents | Previously published equations overestimated REE.A new predictive equation was established for REE in obese children. | (45) |
| Italy | Maffeis et al. | 130 obese and nonobese prepubertal children | Most of the developed equations overestimated REE in obese subjects and equations were generated. | (43) |
Therefore, critically ill children may be at the risk of under/overfeeding with the estimation of energy requirement based on the development of predictive equations (i.e., WHO, Schofield and Harris-Benedict) and stress correction factors (31). Therefore, it is highly recommended assessing energy requirements with indirect calorimetry as a standard method in terms of accuracy in these critically ill children with obesity (15, 20, 21).
After the determination of energy requirement for these obese patients, the administration of major substrates including carbohydrates, proteins, and lipids should be based upon their metabolism in accordance with the nature and phase of the acute illness (15, 17).
3.3. Micronutrient Requirement
Similar to all patients admitted to PICUs, obese children should be assessed for deficiencies in micronutrients and their RDAs amount (10). Children with a critical illness are at increased risk of vitamin B-1, B-2, and B-6 deficiencies (48). Although obesity in children may lead to vitamin B-12 deficiency, the dietary assessment of obese patients before admission in addition to laboratory assessments should be considered in PICUs (49). Some studies reported that high vitamin B-12 level is associated with high inflammation and mortality rate in critically ill adult patients (50). More studies are needed to identify the state of vitamin B-12 in critically ill obese children.
Most critically ill patients are at the increased risk of vitamin A deficiency development according to their nutrition and metabolic states. The deficiency can be severe in patients with lower hepatic retinol storage that probably occurs in obese patients due to recent dietary restrictions for weight reduction such as skimmed dairy products and eggs as two of the best sources of retinol (50).
Magnesium and vitamin D deficiencies are common in obese children and lower 25(OH) vitamin D and magnesium levels in serum are associated with hyperglycemia and insulin resistance in them (30, 51). Magnesium and vitamin D supplementation may be used as an effective, preventive, and therapeutic treatment in the management of obese patients in PICUs (15, 51).
As a cellular antioxidant, vitamin E plays an important role in oxidative stress status. However, insufficient data are available regarding the determination of the role of vitamin E in such patients (50). Therefore, the best way for the diagnosis of vitamin E state is the adjustment of α-tocopherol to total lipids because of different lipid distributions in critical obesity (50). Serum concentrations of zinc and selenium may decrease in PICU settings in response to metabolic pathways in systemic inflammation, but there is incomprehensive data in this regard (50).
Although obesity is associated with the increased risk of iron deficiency anemia, screening for iron deficiency is not recommended in critically ill children since the reduction of serum iron level is determined as a protective response against infection and inflammation in acute phase injury (52). However, according to a study, the hepcidin mass spectrometry dosage method is recommended (53). Key nutritional aspects in the management of children with obesity and critical illness are shown in Table 2.
| Step of Nutrition Support | Recommendation | References |
|---|---|---|
| Nutritional assessment | Anthropometric parameters | (21, 22) |
| MAC | ||
| Bodyweight | ||
| Height/length | ||
| BMI | ||
| Nitrogen balance | (17) | |
| Pre-albumin | (17) | |
| CRP | (22, 27) | |
| Possible/potential laboratory abnormalities | ||
| Complete blood count | (15, 30) | |
| Glucose | (13, 15) | |
| Phosphorus | (15) | |
| Lipid profile | (15, 25) | |
| Total cholesterol | ||
| HDL cholesterol | ||
| LDL cholesterol | ||
| Triglycerides | ||
| Liver function tests | (15, 28, 29) | |
| AST | ||
| ALT | ||
| Direct bilirubin | ||
| GGT | ||
| Energy requirement | Indirect calorimetry is recommended | (15, 17) |
| Macronutrient requirements | Based upon carbohydrate, protein, and lipid metabolism depending on the nature and phase of acute illness | (10, 15, 17) |
| Micronutrient requirements | Routines in RDAs amount | (10, 15, 17) |
| Assessment of possible/potential micronutrients deficiencies and treatment of documented deficiencies | ||
| Vitamin B-1, B-2, and B-6, B-12 | (48-50) | |
| Vitamin A | (50) | |
| Vitamin D | (30, 51) | |
| Magnesium | (15, 51) | |
| Zinc | (50) | |
| Selenium | (50) |
Abbreviations: ALT, alanine amino transaminase; AST, aspartate aminotransferase; BMI, body mass index; CRP, C reactive protein; GGT, γ-glutamyl transpeptidase; MAC, mid-upper arm circumference.
4. Conclusions
In addition to the necessity of preventive strategies for the reduction of childhood obesity prevalence, nutrition planning for the management of obese children admitted to PICUs is of high importance. Optimal energy and nutrient delivery can play an important role in the clinical outcomes of critically ill obese children. These patients constitute a nutritionally high-risk group for whom initial nutritional assessment with suitable nutrition care programs is necessary.
Optimal isocaloric feeding with adequate macronutrients and micronutrients should be administered in critically ill obese children. Since hypocaloric feeding is not recommended in critically ill obese children due to increased catabolism in the metabolic stress state, assessing energy requirement by indirect calorimetry may prevent overfeeding/underfeeding in obese children in PICUs. Further clinical studies, particularly randomized clinical trials, are necessary for the development of nutritional principles in energy requirement, macro/micronutrient delivery, and necessary supplementations.