1. Background
Opium poisoning is a common and fatal poisoning in children. Parallel with an increase in production and availability of methadone in Iran, poisoning from this drug has increased (1, 2). The only approved antidote is naloxone and the best route is intravenous, however, this route is difficult and time wasting, mainly in children (3). The IN route is a clinically effective method for a number of medications in adults and pediatrics, including pain control, anxiolysis, and seizure control (sufentanil, midazolam, ketamine, and dexmedetomidine, etc.), with lots of advantages including rapid onset, high plasma bioavailability, direct transport to CNS across the high vascularization of the nasal mucosa, bypass the first pass metabolism effect, needleless, decreased pain and anxiety for IV catheterization, inexpensive, more tolerable and easy to deliver, and saves valuable staff time (4, 5). In addition, intravenous access in children may be difficult mainly in infants and critically ill patients for inexperienced providers (5). There were two types of administration of IN drugs explained in the literature: drop instillation or by a mucosal atomizer device (MAD) with better bioavailability, however, unfortunately we don’t have Narcan Nasal Spray in our country (6). Nasal discomfort, vomiting and unpredictable dose of IN delivery are the common side effects (3, 7). There are several reports that intranasal naloxone can be safely administered for the reversal of opioid intoxication in the pre-hospital and hospital settings in adults (4).
2. Objectives
According to the lack of enough evidence for IN naloxone in treatment of opioid poisoning in children, this study was designed to evaluate the effectiveness of intranasal (IN) naloxone in opioid poisoning in children as a good alternative method for treatment of this serious poisoning.
3. Methods
3.1. Study Design
This is a prospective, nonrandomized clinical trial study done on children between the ages of one to 13 years with opioid poisoning in Loghman-Hakim Hospital, Tehran, Iran from April 2018 to April 2019. The inclusion criteria were defined as opioid poisoning, between the age of one to 13 years, no severe symptoms, and paternal agreement. Patients with nasal congestion, history of severe poisoning like cardiopulmonary arrest, coma, apnea, cyanosis, and co-ingestion were excluded from the study. The patients who met the inclusion criteria after parents filled the prepared informed consent forms were divided into two groups alternately; Intra nasal naloxone 0.01 mg/kg dribbling (case group) from naloxone ampule, and Intra venous naloxone 0.01 mg/kg injection (control group). Patients with nasal congestion, history of severe poisoning like Cardiopulmonary arrest, coma, apnea, cyanosis and co-ingestion excluded from the study. This study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences (IR.SBMU.RETECH.REC.1397.258) and is registered with the Iranian Registry of Clinical Trials (www.irct.ir) (IRCT20180226038869N1).
3.2. Data Collection
Outcomes included changes in level of consciousness (Pediatrics GCS), time to response, respiratory rate, O2 saturation before and after naloxone administration, side-effects, and need for re-administration.
3.3. Statistical Analysis
Quantitative variables were compared using the Independent t-test. Qualitative variables were compared using χ2 and Mann-Whitney tests. Data were analyzed using SPSS version 17.0 with P < 0.05 being considered statistically significant.
4. Results
4.1. Study Population
A total of 44 patients (22 IV and 22 IN) were enrolled in the study. The mean age was 38.2 ± 28.8 months in the IV naloxone group and 36.8 ± 19.7 months in the IN naloxone group. There was 14 methadone and eight opium poisoning in the IV group and 13 methadone and nine opium poisoning in IN group, all of them were unintentional via oral ingestion. The male/female ratio was 14/8 in both of our study groups (IV and IN). Age (P = 0.85), gender (P = 1), opioid agent (opium and methadone) (P = 0.23); route of poisoning before naloxone administration between the two groups were not significantly different.
4.2. Outcomes of Our Study
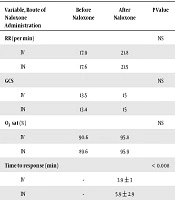
Mean respiratory rate increment before and after intervention was 3.68 ± 3.04 in the IV group and 4.13 ± 2.29 in the IN group, was statistically not significant (P = 0.74). There were no statistically significant differences between the two groups in RR, GCS and O2 saturation before naloxone administration (P = 0.74, P = 0.91, P = 0.22). The IV and IN groups had similar average increases in O2 saturation and GCS after naloxone administration (Table 1).
In total, clinical response to IN naloxone was observed in all of our patients. There was a significant difference in the time of response between IN and IV groups (P < 0.008), 3.9 ± 3 minutes in IV group and 5.9 ± 2.9 minutes in the IN group. Nine patients (41%) in the IV and 20 (91%) in the IN group required further doses of naloxone, which had a statistically significant difference (P < 0.001).
The IV and IN groups had similar average increases in O2 Saturation and GCS after naloxone administration.
| Variable, Route of Naloxone Administration | Before Naloxone | After Naloxone | P Value |
|---|---|---|---|
| RR (per min) | NS | ||
| IV | 17.9 | 21.8 | |
| IN | 17.6 | 21.5 | |
| GCS | NS | ||
| IV | 13.5 | 15 | |
| IN | 13.4 | 15 | |
| O2 sat (%) | NS | ||
| IV | 90.6 | 95.8 | |
| IN | 89.6 | 95.9 | |
| Time to response (min) | < 0.008 | ||
| IV | - | 3.9 ± 3 | |
| IN | - | 5.9 ± 2.9 |
Respiratory Rate, O2 Saturation, and GCS in Opioid Poisoning in Patients Before and After Naloxone Administrationa
5. Discussion
Although we have not found any related article about IN Naloxone in children, there are some articles about the IN route in children. Warrington et al., reported that the IN route is a quick, painless alternative to more invasive routes for pediatric pain management, with similar time to the clinical effect compared to the intravenous route, while minimizing anxiety in both patients and their parents (8). AlSarheed reports the effectiveness of intranasal (IN) sedatives to achieve conscious sedation during dental procedures amongst children and he found that the onset of action for IN midazolam was five to 15 minutes, which is longer than IV; their results about onset was similar to our results (9). Krieter et al., showed the effectiveness of the Aptar Unit-Dose device IN naloxone and that intranasal naloxone resulted in plasma concentrations greater than those observed following the intramuscular dose (10). Madah-Amiri et al., showed successful reversals of opioid overdose with naloxone sprays, however, their research was in adults and Naloxone nasal spray, nonetheless we used IN naloxone dribbling in children (11).
The result of our study regarding RR and GCS were similar to Sabzeghabaee’s result in adults (4). Our study showed that among opioid (opium and methadone) poisoned children, IN naloxone is effective, however, with a short time delay in comparison to IV naloxone. Although our results showed no significant difference between the two groups after naloxone administration in the level of consciousness and respiratory rate increment, O2 Saturation was higher in patients administered IN naloxone than those in the IV group.
Merlin et al., reported in 344 patients who received naloxone by paramedics; changes in RR was six for the IV group and four for the IN group and changes in GCS was four for the IV group and three for the IN group. In our study changes in RR was 3.9 for both the IV and IN group and changes in GCS was 1.5 for the IV group and 1.6 for the IN group (12). Strang et al. mentioned that nasal naloxone has recently been approved and will soon be field-tested, however, buccal and sublingual naloxone is unclear (13).
In our study response time in the IV and IN group were 3.9 ± 3 and 5.9 ± 2.9 minutes respectively, however, McDermott et al., reported that the mean time taken for the IN and IV group was 87.1 seconds and 178.2 seconds, respectively, which may be due to the fact that they added the time needed for IV catheterization (14).
Disadvantages are not extensive; including nasal irritation, restricted delivery volume (max; 1 mL per nostril), and nausea; we had no complications in our study (15).
In addition, the use of this needleless route is effective in the treatment and minimizing the risk of blood-borne exposures to EMS personnel, mainly in addicted patients (16, 17).
5.1. Conclusions
This study showed that the administration of intranasal naloxone is a safe, effective, and well tolerated needleless method for treatment of opioid poisoning in children. Intranasal naloxone has been shown to have many advantages mainly in children both in prehospital, EMS setting, and hospital setting. Although the time to response was longer in the IN groups, if we add the time needed for the IV catheter insertion it seems that the IN route is faster. There are also some limitations to our study, we excluded young infants, critically ill patients, and our study population was not large. Authors recommended similar studies with a higher naloxone dose, large population number, study comparing intranasal and IM or SQ routes, use of other forms of intra nasal medications, and multicenter studies for future researches.