1. Background
Nitric oxide is a substantial signaling molecule produced by airway cells (and other types of cells), which depends on several processes in the airways, such as ciliary activity, ion transport, host defense, inflammation, and vascular tone (1).
Cystic fibrosis (CF) patients often have respiratory tract infections and bacterial colonization that require antibiotic therapy. Pseudomonas infection and a number of previous admissions have shown to be the major predictors of mortality in CF (2-4). The most common organisms causing respiratory infections in CF patients are pseudomonas aeruginosa, staphylococcus aureus, and stenotrophomonas maltophilia (5). In many CF patients, colonization and respiratory infection may not respond appropriately despite antibiotic therapy. Therefore, in these patients, it is necessary to confirm the relief of respiratory infections by using the sputum culture or throat culture and having negative culture results. However, performing the culture of sputum or pharyngeal swab is a time consuming and precise task, in which environmental contamination during sampling or errors in microbial culture can reduce the accuracy of culture. Using alternative and easier methods can be beneficial in monitoring the treatment of CF patients.
The fractional exhaled nitric oxide (FeNO) is reduced in CF patients compared to healthy subjects; and today it has been found that FeNO has an inverse relationship with the airway clearance index (6, 7).
Although studies have shown the role of some infections in changes of FeNO levels, the role of respiratory infections in FeNO has not yet been completely studied (8-15). FeNO measurements are routinely used in the monitoring of stable asthma (16). It is not clear exactly why FeNO is reduced in CF patients. There are some hypotheses in this regard, including the trapping of localized nitric oxide produced in thick mucus, which is one of the characteristics of airways in CF patients as well as reducing the nitric oxide production due to reduction of nitric oxide synthase and increasing consumption of nitric oxide or impairment of ciliary activity (1).
The exhaled nitric oxide in patients with primary ciliary dyskinesia (PCD) is very low, and nasal exhaled nitric oxide is recently considered a valid marker for the diagnosis of this disorder (17-20). Therefore, FeNO measurement as a mucociliary clearance marker (and other pulmonary defects) in CF patients can be used.
2. Objectives
The aim of this study was to investigate whether FeNo is a non-invasive test to use for evaluation of treatment in CF patients.
3. Methods
This cross-sectional case-control study was conducted on CF patients from the age of five to 18 with positive sputum culture, through simple non-random census method, in which 30 CF patients referring to the children’s hospital were selected. Patients under five years were excluded from the study due to their inability to perform the FeNO measurement technique. If the patient did not have sputum or throat culture, through the standard technique a sample would be taken from him/her. If there was sputum culture during the last week, it would be considered as the culture. Generally, the inclusion criteria included individuals between the age of five and 18, affection of cystic fibrosis, and positive sputum culture. The exclusion criteria included patients who could not do a FeNo test, use of systemic corticosteroid, starting antibiotic therapy within 72 hours before FeNo test and had a positive culture after antibiotic therapy. If a subject had sputum or throat culture, he would remain in the study. Sputum culture results, including the growing organism and antibiogram response, were recorded in the patient datasheet. At the same time, 30 healthy children were selected as the control group. Patient’s demographic data was obtained through interviews. The patient’s weight was measured by the researcher using seca scales (in minimal clothing). The patient’s parents were asked about the information on the patient’s medications, the ways to clean the airways, the use of the hypertonic saline nebulizer, nebulized N-acetylcysteine (NAC), and the use of inhaled and systemic corticosteroids. The information was recorded in the patient datasheet. Before starting the treatment, the FeNO levels of all subjects (both the case and the control group) were measured by the researcher using the NO-Meter device, single-breath technique, and oral method, which was based on the American Thoracic Society (ATS) guideline. The results were registered in the datasheet. The test was performed for each patient twice with a one minute interval and the mean was recorded based on ppb. The test was performed in a sitting position at room temperature. The patient was fasting from one hour before the test. For patients, standard antibiotic therapy was performed based on antibiogram and the physician’s diagnosis (pediatric lung specialist). If the antibiotic therapy started more than 72 hours before the FeNO was measured patients would be excluded from the study. Culturing was performed again two weeks after the initiation of treatment. Two weeks after starting the treatment, FeNO was measured with the same device; and for patients who were referred four weeks after the initial visit, FeNO was re-measured in the same manner. The results were entered in the data collection sheet. Finally, the data was entered in the SPSS software and analyzed.
After data entry, data analysis was performed in SPSS (version 23). The central tendency and the mean of variables were analyzed; Wilcoxon signed-rank test and Mann-Whitney U-test were used for analysis.
4. Results
In the present study, 30 CF patients who had a positive sputum culture as well as 30 age-matched healthy children were included. In the case group, 27 out of 30 patients stayed in the study (27 subjects who had negative cultures, after two weeks of taking antibiotics).
In the case group, 14 subjects (46.7%) were female, while in the control group 16 subjects (53.3%) were female. The statistical difference between both groups was not significant in terms of gender (P = 0.797).
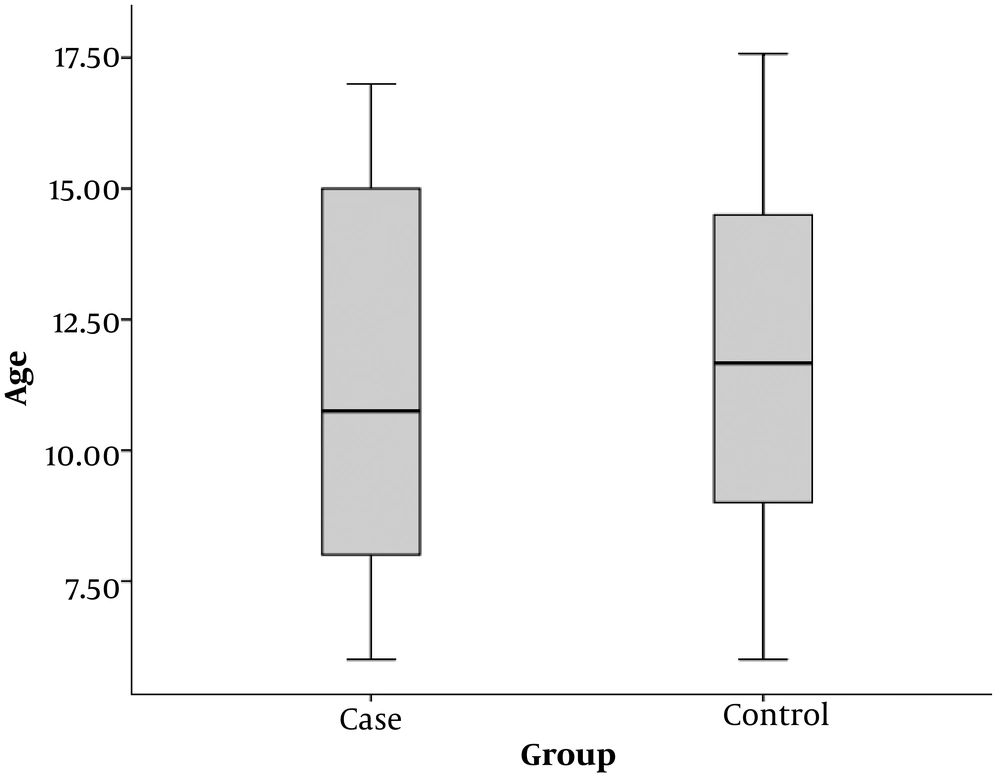
The mean age of children in the case group (patients with positive sputum culture) was 11.4 ± 3.8 years and the mean age of children in the control group was 11.7 ± 3.2 years. There was no significant difference in the mean age between both groups (P = 0.705) (Figure 1).
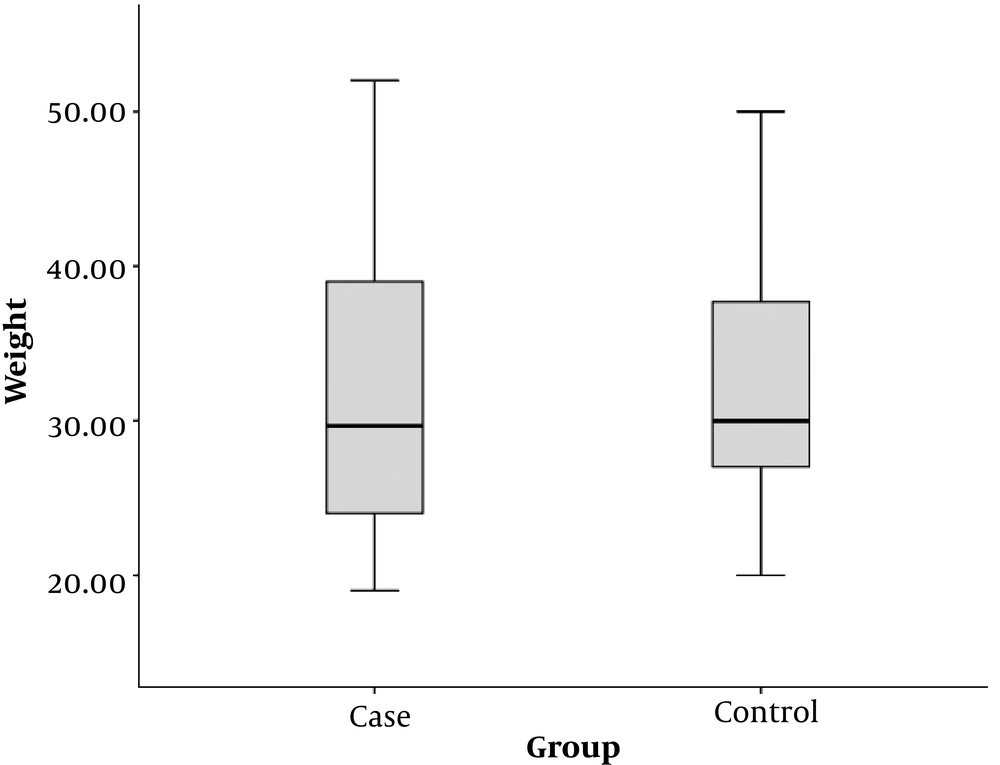
The mean weight of children in the case group was 31.9 ± 10.0 kg and the mean weight of children in the control group was 32.1 ± 7.2 kg. There was no significant difference in the mean weight between both groups (P = 0.944) (Figure 2).
Three patients (10%) received daily oral NAC. A total of 24 patients (80%) used a salbutamol nebulizer daily; and 23 subjects (76.7%) used inhaled steroids. In the daily treatment plan, all 30 patients had nebulized hypertonic saline. The results of sputum culture in the case group were as follows:
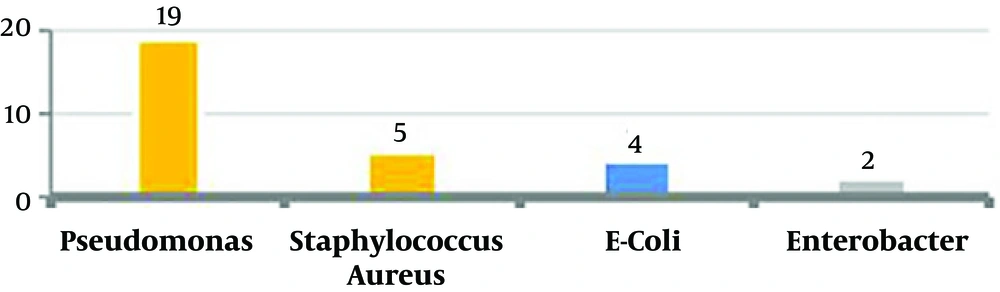
In 19 subjects (63.3%) there were pseudomonas; five subjects (16.7%) had staphylococcus aureus, four subjects (13.3%) had Escherichia coli, and two subjects (6.7%) had Enterobacter (Figure 3).
Two weeks after starting antibiotic treatment, the results were negative in 27 cases; and sputum culture still remained positive in only two patients (10%). All these two patients were positive for pseudomonas culture and excluded from the study.
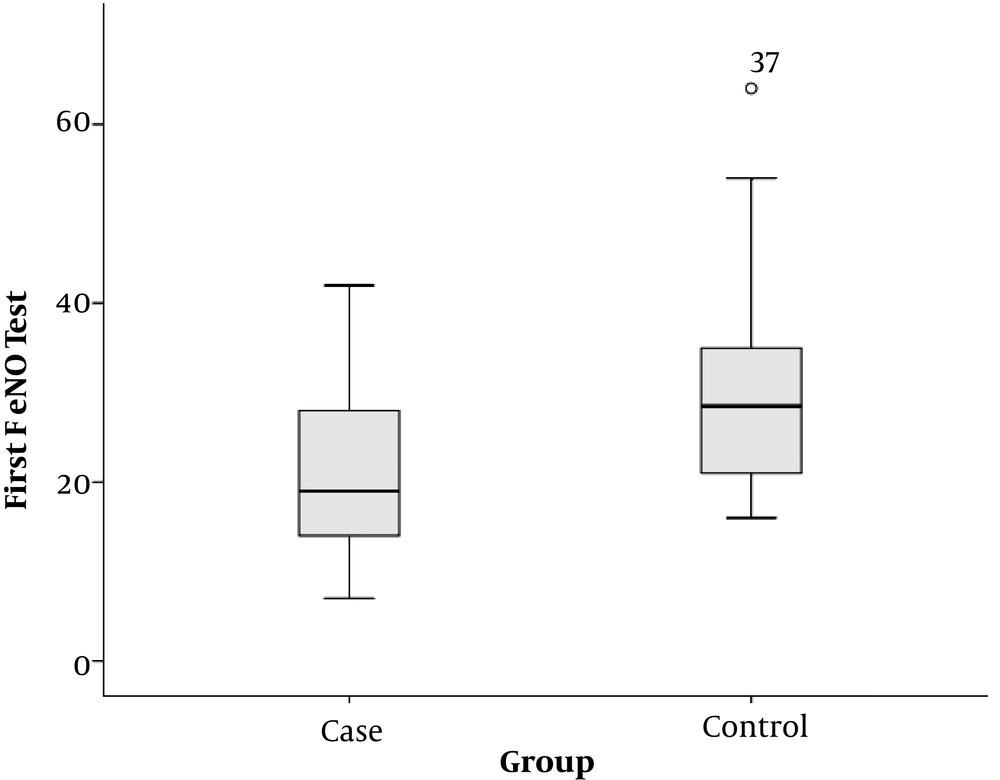
The mean of recorded FeNO at the beginning of the study was 22.4 ± 10.1 in the case group and 30.0 ± 11.0 in the control group. This difference was statistically significant and the subjects had lower levels of FeNO in the case group than in the control group (P = 0.003) (Figure 4).
In the case group, there was no significant association between the weight and initial FeNO level (P = 0.292). Moreover, in this group, there was no significant relationship between the age and initial FeNO level (P = 0.110).
In the case group, the mean of recorded FeNO at the beginning of the study was 22.4 ± 10.1, and two weeks after starting the antibiotics treatment, it was 16.4 ± 5.5. FeNO changes in patients before and after antibiotic therapy were -6.0 ± 9.4 (ranging from +7 to -33).
There was a significant difference between FeNO before treatment and after two weeks of antibiotic treatment; FeNO levels decreased after two weeks of antibiotic therapy in the case group (P = 0.003).
Four weeks after the initial visit, the FeNO test was repeated in the same manner for 13 patients in the case group (two weeks after antibiotic therapy).
The mean of recorded FeNO for these 13 patients at the beginning of the study was 24.38 ± 8.38; while it reached 18.85 ± 6.44 two weeks after starting antibiotic therapy, and was 20.08 ± 7.41 two weeks after discharge (four weeks after the initial visit). There was no significant difference between the FeNO levels two weeks after starting antibiotic therapy and four weeks after the therapy (P = 0.292).
After two weeks of antibiotic therapy, 16 out of 27 patients with negative sputum culture had pseudomonas and 11 patients had non-pseudomonas in their first culture.
The initial FeNO level in subjects with pseudomonas culture was 24.9 ± 11.6 and in subjects with non-pseudomonas culture, it was 18.7 ± 6.1. There was no significant difference in the initial FeNO levels between subjects with pseudomonas culture and subjects with non-pseudomonas culture (P = 0.084).
The mean FeNO after two weeks of antibiotic therapy in subjects with pseudomonas culture was 16.7 ± 6.3 and in subjects with non-pseudomonas culture 15.9 ± 4.4. There was no statistically significant difference in the FeNO levels following the antibiotic treatment in subjects with pseudomonas culture and subjects with non-pseudomonas culture (P = 0.726).
FeNO changes in patients with pseudomonas culture were -8.3 ± 11.5 and in patients with non-pseudomonas culture were -2.8 ± 3.7. There was no statistically significant difference between subjects with pseudomonas culture and subjects with non-pseudomonas culture in terms of FeNO change before and after the treatment (P = 0.094).
5. Discussion
This study has shown the role of FeNo test in children with cystic fibrosis and its association with sputum culture.
We examined 27 CF children and 30 healthy children. In the present study, the mean of recorded FeNO at the beginning of the study was 21.63 ± 9.98 in the case group and 29.97 ± 10.98 in the control group. This difference was statistically significant, and CF patients had a lower FeNO level than the subjects in the control group (P = 0.003). Similar results have been reported in most other studies (21, 22).
In general, the reasons for the reduction of FeNO in CF patients included the reduction of NO production due to decreased NO synthase activity either in nature or as NO-induced reduction, an increase in levels of ADMA, which was an endogenous inhibitor of NO synthase activity in the sputum of CF patients, an increase in NO catabolism, and mechanical factors such as thick discharge that acted as the barrier against NO release, etc. (21).
FeNO levels in our study did not correlate with the type of organism, and the level of initial FeNO in subjects with pseudomonas culture was 24.9 ± 11.6 and in subjects with non-pseudomonas culture was 18.7 ± 6.1. There was no significant difference in the level of initial FeNO between subjects with pseudomonas culture and subjects with non-pseudomonas culture (P = 0.084).
In the studies done by Hofer et al., and Kotha et al., there was no association between FeNO and type of organism (22, 23).
Benjamin Gaston et al., in 2002, published their article on nitrogen redox balance in CF patients. They only studied CF patients with pseudomonas colonization and excluded the patients who had colonization with other organisms. In the present study, the FeNO levels in patients increased after treatment with anti-pseudomonas, which was statistically significant (P < 0.05) (24).
Jaffe et al. in 2002 evaluated the effect of antibiotic therapy on FeNO in a number of CF children. The mean FeNO at the beginning of the study was 5.8 ppb (2.0 - 14.3), which reached 9.2 ppb (0.8 - 25.1) after the antibiotic treatment (P < 0.05). In the study of subgroups, FeNO changes were not impressive in the group with positive pseudomonas culture. Besides, no significant association was found between lung function (spirometry findings) and FeNO (7). In our study, after two weeks of initiation of antibiotic therapy, the mean FeNO in the case group decreased from 22.4 ± 10.1 to 16.4 ± 5.5. FeNO reassessment was performed for 13 patients two weeks after discharge (four weeks after beginning the study). There was no significant difference in FeNO levels between two weeks after initiation of antibiotic therapy and four weeks after the administration of antibiotics (P = 0.292). In some studies, similar results have been obtained in line with our study, for instance, in the study of Kotha et al., they conducted a study to evaluate the use of FeNO as a biomarker for the CFTR function. In one part of the study, 34 CF patients who were hospitalized with exacerbation diagnosis were included. For these patients the FeNO levels were measured in the first 72 hours after admission, prior to discharge, one month after discharge, and two to four months after discharge. The levels of FeNO in these patients did not show any significant difference before and after treatment and discharge. In addition, there was no significant relationship between gender, age, type of micro-organisms obtained from sputum culture, and FeNO level (23). Jobsis et al., studied hydrogen peroxide and nitric oxide in the CF children’s expiratory air after antibiotic treatment. In their study, following the CF exacerbation, 16 CF children with a mean age of 12.3 years were administered by antibiotics. During the treatment, the level of hydrogen peroxide was measured twice a week. Serial FeNO measurements were performed for nine patients. FeNO was assessed in two techniques for patients. Injectable antibiotic treatment was administered based on the susceptibility of cultured micro-organisms and respiratory physiotherapy for two to five weeks (average of three weeks). The concentration of H2O2 was significantly reduced by antibiotic therapy and dropped from 0.28 μM to 0.16 μM (P = 0.002); however, there was no significant difference in expiratory nitric oxide levels following the treatment (P = 0.4) (25). In the study of Ho et al., FeNO levels in adults with cystic fibrosis did not significantly differ after the antibiotic therapy (26).
5.1. Conclusions
The precise cause of the contradiction between our study and Jaffe’s study is unknown. However, it may be justified for the following reasons: difference in sample size, not considering the severity of pulmonary involvement in subjects, and technical errors in performing the FeNO test.
In sum, by comparing the present study and the results of similar studies, it can be concluded that oral FeNO cannot be used as a method for replacing sputum culture in monitoring CF patients.
5.2. Limitations and Suggestions
Patient’s disregard for referral at the specified time was the biggest limitation of this study, thus, in many cases we had to call patients to refer for visit. In addition, some patients failed to cooperate in the preparation of a suitable sputum sample. Moreover, during the project the FeNO meter was broken, and lack of any alternative device caused a gap in project.
We suggest that the following points should be taken into consideration to design a similar study:
- Determining a larger sample size;
- Considering the extent and severity of lung involvement in each patient;
- Using a spirometry if possible;
- Measuring simultaneously by the second device and comparing the accuracy of both devices.