1. Background
Hemophilia is a rare X-linked inherited bleeding disorder characterized by the deficiency of coagulation factor VIII (hemophilia A) or IX (hemophilia B) (1). Consequently, it is accompanied by repeated bleeding episodes, including hemarthrosis leading to reduced mobility (2). Novel treatments and better care have led to higher life expectancy in patients with hemophilia, thereby increasing the risk of age-related complications such as osteoporosis (3). Previous studies reported the prevalence of osteoporosis in hemophilia patients to be 26.9% in Greece (4), 34% in Turkey (5), 27% - 38% in the United States (3, 6), 28.6% - 35.7% in Iran (7-9), and 16.7% in Egypt (10). The most frequent reported associated factors were vitamin D deficiency (6, 11), hepatitis C infection (6, 11-13), and low physical activity (6, 10, 14, 15); however, there is some inconsistency between the prevalence of osteoporosis and the associated risk factors in patients with hemophilia in different countries (6, 10, 12-14).
2. Objectives
Due to the conflicting results on the prevalence and risk factors of low bone mass in previous studies, the current study aimed to evaluate bone mass and its related factors in patients with hemophilia in Southern Iran. Understanding this information can help prevent osteoporosis and fracture in these patients.
3. Methods
This cross-sectional study was conducted on 120 patients with hemophilia aged 9 - 82 years. They were routinely visited and followed up in the Dastgheib Hemophilia Clinic affiliated with Shiraz University of Medical Sciences, Iran, from April 2017 to September 2018.
The patients were classified into three groups according to the activity of factor VIII or IX levels as follows: mild: a factor activity > 5% - 40% of normal; moderate:1-5% of normal; and severe: less than 1% of factor activity in the healthy population which corresponds to < 0.01 IU/mL. The exclusion criteria were the patients with diabetes, chronic renal failure, other metabolic bone diseases, such as rickets and congenital skeletal dysplasia, use of anti-convulsant medications, and history of physical impairment, which restricted normal ambulation. The results of the hepatitis C virus (HCV) seropositivity and PCR analysis were recorded for each patient. Five patients were excluded, and four patients refused to participate in this study. Finally, 111 patients participated in the present study.
3.1. Ethical Considerations
The present study was approved by the local Ethics Committee of Shiraz University of Medical Sciences, and the Vice-Chancellor of Research approved under the code of 97-01-01-17020. We obtained the written informed consent from each patient.
3.2. Anthropometric Measurements, Body Mass Index, Physical Activity, and Sun Exposure
An expert physician measured the height, weight, and pubertal stage of patients according to standard scales and protocol while the patient wore light clothes without shoes. Body mass index (BMI) was calculated as:
BMI (kg/m2) = weight (kg)/[Height (m)]2.
The categorization of physical activity was based on the American College of Sports Medicine recommendation (16, 17). Sufficient physical activity was defined as having more than three days of exercise per week (16). Those who had more than 30 min/day exposure to sunlight (18).
3.3. Biochemical Studies
We used the Biosystem autoanalyzer, Spain, to measure serum calcium (Ca), phosphorous (P), and Alkaline phosphatase. Serum 25-hydroxy vitamin D (25OHD) was measured with the electroluminescence method. Vitamin D deficiency was labeled if serum vitamin D level was below 20 ng/mL (19).
3.4. Bone Densitometry
Hologic system dual-energy X-ray absorptiometry (DXA) (discovery QDR, USA) was used to evaluate bone mineral density (BMD). The coefficient of variation in our center was 0.5% for the lumbar spine and 2.5% for the femur, based on the measurements in ten people. We defined low bone mass (LBM) for chronological age as having BMD Z-score less than -2 for patient’s age and gender.
3.5. Statistical Analysis
SPSS software, version 21, was used for data analysis. Descriptive data were written as mean ± SD and percentage. Quantitative data were compared using the student's t-test and Mann-Whitney U-test. Also, the qualitative data were analyzed using the chi-square and Fisher exact tests. A binary logistic regression test was used to evaluate confounding factors.
4. Results
One hundred and ones patients with hemophilia aged 32 ± 12.9 years participated in our research, of whom 96.4% were male, 83% had hemophilia type A, 17% had hemophilia type B. Concerning the serum level factors, 42% of patients had severe factor deficiency (< 1% of the factor was present in their blood sample), and 44% had moderate factor deficiency (1% - 5% of the factor was present in their blood sample). About 3.6% of the patients had a positive PCR test for HCV. Table 1 shows the general characteristics and laboratory data.
| Variable | Values |
|---|---|
| Age, y | 32 ± 12.9 |
| Weight, kg | 64 ± 15.1 |
| Height, cm | 169.7 ± 12 |
| BMI, kg/m2 | 22.1 ± 4 |
| Gender (M/F) | 107/4 |
| Type of disease, % | |
| Hemophilia A | 82.9 |
| Hemophilia B | 17.1 |
| Ca, mg/dL | 9.5 ± 0.5 |
| P, mg/dL | 3.4 ± 0.76 |
| Alk, µU/L | 269 ± 126 |
| 1,25(OH)2D3, pg/mL | 25.1 ± 16.9 |
| PTH, pg/mL | 43.2 ± 23.6 |
| TSH, mIU/L | 2.1 ± 1.6 |
| Sun exposure, % | |
| < 30 min | 49.5 |
| > 30 min | 50.5 |
| Physical activity, % | |
| < 3 | 77.4 |
| ≥ 3 | 23.6 |
| The severity of factor deficiency, %b | |
| Severe | 42 |
| Moderate | 44 |
| Mild | 14 |
| HCV infection, % | |
| Negative | 75.7 |
| Curec | 20.7 |
| Positive | 3.6 |
General Characteristics and Laboratory Data of Patients with Hemophiliaa
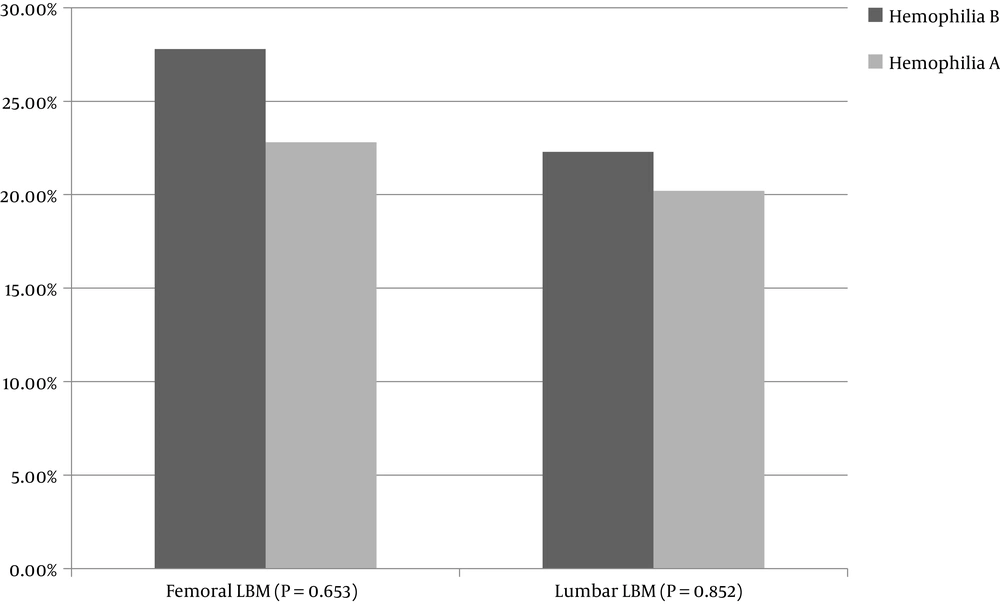
The prevalence of low bone mass for chronological age was 20.6% in the lumbar area and 23.7% in the femur. Between hemophilia A and B, no significant difference was observed in low bone mass in the femur or lumbar area (P = 0.653 and P = 0.852, respectively). Figure 1 shows the prevalence of LBM in the femur and lumbar regions in hemophilia A and B.
Univariate analysis showed that LBM was associated with body weight (P = 0.044), height (P = 0.041), and severity of factor deficiency (P = 0.016). However, binary logistic regression analysis showed that LBM was merely associated with the severity of factor deficiency (P = 0.037, beta = 0.896). In univariate analysis, low femoral bone mass was associated with body weight (P = 0.004), BMI (P = 0.023), and BMI percentile (P = 0.028). However, binary logistic regression test showed that low femoral bone was associated with severity of factor deficiency (P = 0.022, beta = 0.939), BMI/BMI percentile (P = 0.013, beta = -0.181), HCV infection (P = 0.012, beta = 1.4), and weight (P = 0.002, beta = -0.064) in femur neck. Table 2 summarizes the results of univariate analysis, evaluating the association of LBM in the femur/lumbar bones and the possible associated factors.
| Low Lumbar Bone Mass | Low Femoral Bone Mass | |
|---|---|---|
| Age | 0.737 | 0.145 |
| Weight | 0.044 | 0.004 |
| Height | 0.041 | 0.112 |
| Calcium | 0.834 | 0.885 |
| Phosphorus | 0.768 | 0.183 |
| PTH | 0.497 | 0.076 |
| T4 | 0.446 | 0.161 |
| TSH | 0.712 | 0.739 |
| Vitamin D | 0.150 | 0.229 |
| ALK | 0.165 | 0.268 |
| BMI, kg/m2 | 0.190 | 0.023 |
| Gender | 0.504 | 0.56 |
| Type of hemophilia | 0.538 | 0.43 |
| Vitamin D deficiency | 0.323 | 0.448 |
| BMI Percentile, % | 0.117 | 0.016 |
| Physical activity | 0.493 | 0.438 |
| Sun exposure | 0.496 | 0.576 |
| HCV infection | 0.788 | 0.011 |
| Severity of factor deficiency | 0.016 | 0.028 |
Association of General Characteristics and Laboratory Data of Patients with hemophilia Considering Low Bone Mass in the Lumbar and Femoral Areasa
5. Discussion
The present study showed that the prevalence of low bone mass in the femur and lumbar bones was 23.7% and 20.6%, respectively, in patients with hemophilia in southern Iran. Low bone mass in the femoral bone was associated with body weight, BMI, the severity of factor deficiency, and HCV infection. However, low bone mass in the lumbar area was only associated with the severity of factor deficiency.
The prevalence of LBM in patients with hemophilia was reported in a broad spectrum of 7.5% (20) to 38% (21) in previous reports. This wide range can be explained by the inhomogeneity of different reports in terms of the severity of hemophilia, the definition of low bone mass (considering T score or Z-score), age, and ethnicity of the studied patients (4). Roushan et al. (8) reported that the prevalence of low bone mass in patients with hemophilia in northern Iran was 23.8% and 14.6% in the spine and femur, respectively, which is in line with our data. LBM in patients with hemophilia was more prevalent than the normal Iranian population, with a prevalence of 3.1% - 10.7%, reported in the previous studies (22, 23).
Previous studies reported some discrepancies in the risk factors associated with low bone mass in patients with hemophilia in different populations. While low physical activity, HCV infection, vitamin D deficiency, BMI, arthropathy, and cigarette smoking were the possible risk factors in previous reports (4, 7, 11, 12, 14, 24-27), our data proved that severity of factor VIII / IX deficiency was the most critical risk factor associated with both femoral and spinal LBM. One animal study showed that, unlike normal mice, non-bleeding mice with hemophilia had lower BMD and abnormal bone structure (26, 28). Hence, it seems that hemophilia, independent of its other complications, such as HCV infection or arthroplasty, is associated with low bone mass (26, 28). This hypothesis is supported by several other studies that showed some thrombin receptors on osteoblasts (26, 28, 29), in addition to the inhibitory effect of factor VIII on osteoclastogenesis (30).
Another finding in the present study was the association of HCV infection with LBM in the femur bone, which was previously observed (7, 8, 12, 25, 26). It is important to remember that a history of HCV infection is more likely to be positive in older patients with severe diseases. However, in the present study, this association only existed in our hemophilic patients in spite of performing adjustment analysis to limit the effect of confounding factors, such as age and disease severity. HCV-associated hepatitis and liver disease might cause low bone mass (26). Moreover, some studies found an increased level of bone resorption markers in patients with chronic viral hepatitis, which had an inverse correlation with BMD (31, 32).
The present study showed that there was an inverse correlation between low bone mass and BMI or body weight. This finding was in line with those of Iorio et al. (12) and Kempton et al. studies (3) showing that for each lower BMI point, BMD declined 0.0009 g/cm2 (3). It is noteworthy that the association of low BMI and low BMD was seen in the general population as well (33). Moreover, some previous reports suggested that vitamin D deficiency and low physical activity might be associated with low bone mass; however, we did not observe such a relationship. This finding might be due to routine vitamin D supplements in our hemophilic patients and patients’ misinterpretation of their physical activity (8).
Despite many strengths of this study, including the large number of hemophilic patients in Southern Iran, we encountered some limitations in this study. For instance, the results could have been more robust if we had a control group from the normal population to compare their BMDs more accurately. Besides, we did not evaluate the effect of arthropathy on the LBM of our patients. It is suggested that future studies be conducted with a control group and an assessment of the arthropathy score of patients with hemophilia.
5.1. Conclusions
LBM is a frequent complication in patients with hemophilia in southern Iran. The severity of the disease, HCV infection and BMI were the most important associated factors.