1. Background
Jaundice is one of the most common problems in the neonatal period and is the clinical manifestation of hyperbilirubinemia (1). In healthy subjects, the concentration of bilirubin in blood plasma is low (< 17 μmol/L). However, this increased in those with jaundice (> 30 μmol/L). Concentrations of bilirubin in infants increase immediately after birth due to high turnover of hemoglobin, which the body is unable to eliminate in the short run (2). Jaundice occurs in the first week of life in approximately 60% of the term and 80% of preterm infants and is one of the main reasons for the hospital referral during the first month after birth (3). Most of the cases are physiologic, but in the case of pathologic jaundice, if not treated, it can lead to serious disorders such as impaired development of the central nervous system (neurotoxicity or kernicterus) (4).
Predisposing risk factors of pathological jaundice include infection or trauma at birth, prematurity or polycythemia and genetic factors (5). Total serum bilirubin measurement (TSB) is the gold standard method for bilirubin measurement. However, since this method is aggressive, painful, and in need of skill, transcutaneous bilirubin measurement (TcB) can be used as an alternative to TSB (6). Transcutaneous bilirubin measurement is a non-invasive, quick and easy method that measures the bilirubin level through the skin and subcutaneous tissues (7, 8). This method predominantly indicates bilirubin in extravascular spaces and is likely to be influenced by skin pigmentation and thickening. Conversely, TSB reflects intravascular bilirubin concentration (9) Although the TcB measurement provides accurate estimates, no correlation was discerned between TcB and TSB during phototherapy; BiliChek and JM-103 bilirubinometers significantly elevate TSB levels in dark-skinned neonates and may lead to unnecessary or extra treatments (10).
To our knowledge, previous studies have also demonstrated a good correlation between TcB and TSB in the measurement of serum bilirubin levels before and after phototherapy (11-13). However, phototherapy can reduce serum bilirubin level, no study has been conducted to compare these two measurement methods during phototherapy after covering the frontal and sternal areas of the infant.
2. Objectives
The aim of this study was to compare serum bilirubin level with two measurement methods of TcB and TSB after covering the frontal and sternal area of the infants.
3. Methods
3.1. Study Design
This descriptive-analytical study was conducted to compare the accuracy of the reported serum bilirubin level of neonates under phototherapy with two methods of TSB and TcB.
3.2. Participants
The study population consisted of eligible neonates admitted to the neonatal ward of Vasei Hospital in Sabzevar, Iran, between Oct 30, 2016 and Aug 7, 2018. The convenience sampling method was performed. The icteric term and late preterm neonates with a total bilirubin levels above 15 mg/dL and gestational age of more than 34 weeks and weight of more than 2200 grams were enrolled in this study in their first 96 hours of life, and those with diseases other than jaundice, the need for exchange transfusion due to high hyperbilirubinemia or direct hyperbilirubinemia were excluded. The informed consent was obtained from all parents whose newborn was admitted to this study. This research was approved by the Ethics Committee of Sabzevar University of Medical Sciences with the code of (IR.MEDSAB.REC.1394.75).
3.3. Sample Size
The sample size was calculated as 200 patients based on effect size, first type error, power, and dropout rate accounting for 0.26, 0.05, 0.95% and 10% using G-power software.
3.4. Measurement Method
During the first 4 days of birth, the TcB and then TSB of the neonates were measured. The neonates with hyperbilirubinemia (bilirubin > than 15 mg/dL) underwent phototherapy. Again, after 12 to 24 hours of phototherapy, as before, first TcB and then TSB were measured. Measurements of TcB and TSB were carried out with two hours’ interval. Measurement of TcB was performed using BiliCheck KJ-8000 Transcutaneous Jaundice Meter, made by Xuzhou Kejian High-Tech Co., Ltd with product registration number of YZB/SU0372/2007 at the forehead between the two eyebrows and the middle part of the sternum. The calibration function of the device is auto and the calibration screen display; “00.0” or “00.1” for “00”. Measuring TSB was performed by a clinical laboratory method using a semi-automated biochemical analyzer on a blood sample taken from the neonatal heel. Infants were placed with a distance of about 20 - 25 cm in the phototherapy devices (made by NeoMedLight® Company), the 8 Tumble branded Tucson lamps (TL 20W/52 bulbs) with wavelengths of 425 - 475 nm, for 24 hours a day for four consecutive days. In addition to shielding the eyes and the genital area, in order to prevent the injury during phototherapy, the sternum and frontal areas were also covered by a white 3 × 3 cm Gauze. All measurements of TcB and TSB were performed by a trained nurse who had working experience in the neonatal department. For each patient, the data on both TcB and TSB, before and after phototherapy, as well as the date and time of the measurements were recorded in an Excel chart. The mean levels of bilirubin of the sternum and frontal areas were recorded as TcB value at each measurement.
3.5. Statistical Analysis
Quantitative and qualitative variables were presented as mean ± SD and frequency (percent), respectively. Correlations of serological-transcutaneous frontal and serological-transcutaneous sternal measurement data were evaluated using Pearson’s correlation (normal distribution), Spearman correlation (abnormal distribution), and intraclass correlation coefficient (ICC), respectively. Bland-Altman test was used to define the agreement of the mean difference between the gold standard TSB and the tested tool TcB. All the analyses were performed using STATA (version 12, Stata Corp, College Station, Texas, USA).
4. Results
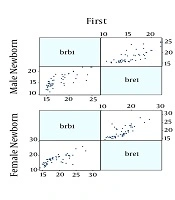
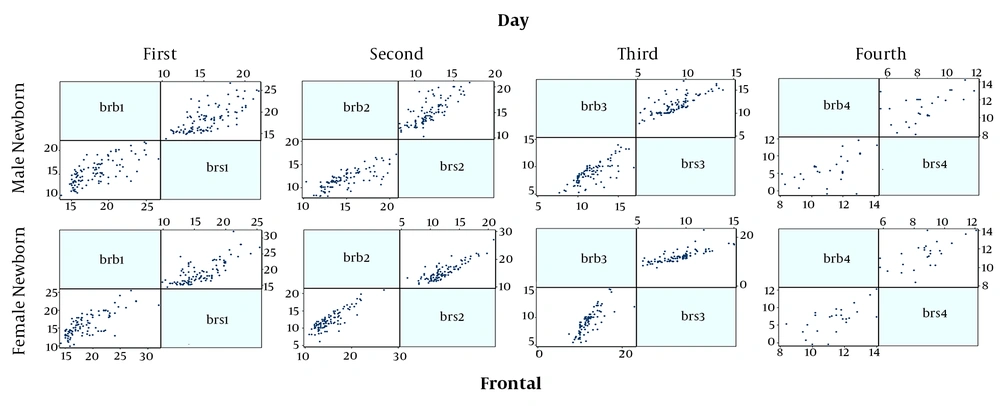
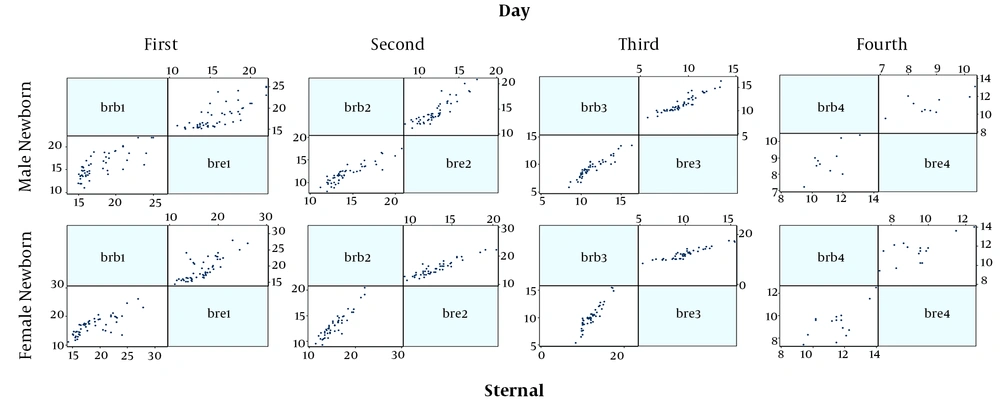
Finally, 230 neonates were enrolled, the mean age of them were 2.90 ± 1.65 days and 50.5% were male and also most of them were in the range group of 15 to 18 mg/dL serum. All the demographic data are summarized in Table 1. Figures 1 and 2 show the correlation between the two serological-transcutaneous frontal and sternal tests, respectively, separated by male and female from the day one to four of birth. As these graphs show, for both male and female infants, there is a greater degree of similarity for the two serological and transcutaneous (frontal and sternal) tests in the lower levels of bilirubin, however, for the serological-transcutaneous sternal tests, there is a more consistent correlation between the data. The correlation for serological-transcutaneous frontal tests was 0.74, 0.82, 0.76, and 0.55 for the first to fourth day, respectively, versus to 0.71, 0.91, 0.92, and 0.69 for the mentioned days for serological-transcutaneous sternal tests. In other words, the correlation between the two tests on the first day that the infants were not under phototherapy was high and it was reduced by phototherapy. Also, the correlation recovery in the serological-transcutaneous sternal test is better than that of serological-transcutaneous frontal tests.
| Parameters | Values |
|---|---|
| Age (days) | 2.90 ± 1.65 |
| Weight (gr) | 3144.75 ± 376.54 |
| Gestational age (weeks) | 38.4 ± 1.42 |
| Gender | |
| Male | 101 (50.5) |
| Female | 99 (45.5) |
| Bilirubin category (mg/dL serum) | |
| 15 - 18 | 100 (50) |
| 18 - 21 | 54 (27) |
| > 21 | 46 (23) |
aValues are expressed as mean ± SD or No. (%).
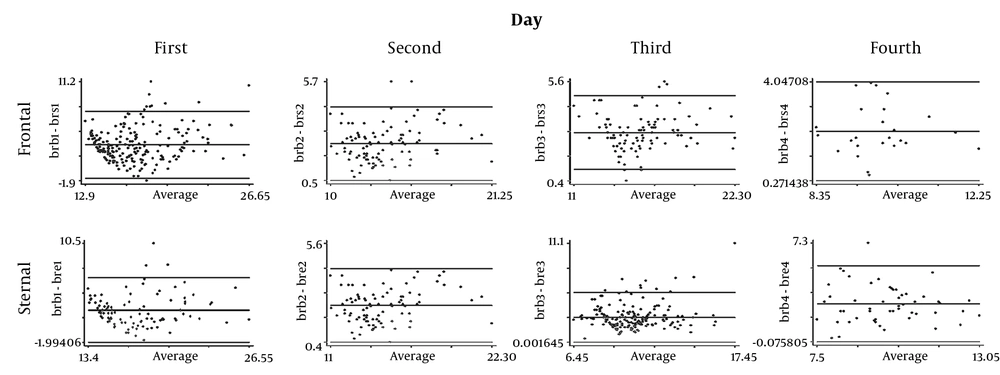
Figure 3 the correlation of the serological-transcutaneous frontal and sternal tests with the Bland-Altman method during four days; although the correlation between the two methods is high on the first day in both areas, the value of reduction is greater on the fourth day in the frontal area.
The correlation between the two serological-transcutaneous frontal and serological-transcutaneous sternal tests using the ICC method, between days one and four are presented in Table 2. As the table shows, the correlation between the serological-transcutaneous frontal tests in the first three days is moderate and the highest correlation was observed on the third day, however, it was sharply reduced on day four. Similar to the serological-transcutaneous frontal tests, the correlation level for the serological-transcutaneous sternal test was moderate but its value was better than that of serological-transcutaneous frontal tests. Also, the correlation level in this test, like the serological-transcutaneous frontal tests decreased on the fourth day, it was moderate, though.
| Time | ICC | MS (Between) | MS (Within) | |||
|---|---|---|---|---|---|---|
| Frontal | Sternal | Frontal | Sternal | Frontal | Sternal | |
| Frist day | 0.41 | 0.60 | 18.90 | 14.30 | 6.50 | 4.30 |
| Second day | 0.37 | 0.60 | 11.20 | 10.20 | 5.90 | 3.50 |
| Third day | 0.81 | 0.50 | 7.10 | 6.50 | 4.90 | 2.45 |
| Fourth day | 0.00 | 0.41 | 3.80 | 2.50 | 4.80 | 2.80 |
Abbreviations: ICC, intraclass correlation coefficient; MS, mean square.
5. Discussion
The aim of this study was to compare the serum bilirubin level of neonates using two methods of serological transcutaneous measurements after covering the frontal and sternal area. In general, the purpose of this study was to answer the question of whether covering the frontal and sternal area could reduce the correlation between the two TcB and TSB methods. The findings of this study showed a moderate correlation between serological and transcutaneous measurement methods before and after phototherapy. The results also showed a greater correlation between serological-transcutaneous sternal versus serological-transcutaneous frontal tests. Overall, the results of this study indicate that TcB could be a good alternative to TSB in its absence.
To the best of our knowledge, this is a first study to investigate the correlation between TcB and TSB with the covered frontal and sternal area. However, the correlation between the two methods of TcB and TSB has been addressed in several studies. The results of a study by Tan et al. showed that the correlation between TcB and TSB in the covered frontal area (r = 0.74) was close to the correlation of the areas under phototherapy (r = 0.60) and the correlation for the frontal area was less than the control group that was not under phototherapy (r = 0.88) (14). In another study by Rylance et al., the results showed that TcB correlation (in both covered frontal and non-covered sternal areas) with TSB in infants under phototherapy (r = 0.66) was lower than those without phototherapy (r = 0.83) (15). The findings of these two studies suggest that phototherapy can affect the correlation between TcB and TSB through the bleaching effects on the skin. In some other studies, the correlation between the two methods for the covered frontal area has been reported higher than our study. These studies include the Rizvi et al. (r = 0.88) (2), Rohsiswatmo et al. (r = 0.87) (16), and Mansouri et al. (r = 0.89) studies (13). High levels of correlation reported by these studies could be related to the different versions of TcB instruments used in these studies.
In the study by Mahajan et al. (17), the correlation between the two methods for the uncovered sternal area was reported r = 0.90, which is more than the value of correlation for the corresponding area in our study. But in studies conducted by Taylor et al. (18) and Tan et al. (14), the correlation for this area was r = 0.78 and 0.60, respectively, which is close to that of our study. Our study also found that the TcB method overestimated the serum bilirubin levels compared to TSB. These results are consistent with the results of the studies conducted by Greco et al. (19) and Murli et al. (20). This can be due to the TcB method standard and tool used for the measurement.
Considering the TcB test has good diagnostic accuracy and is a reliable tool to assess bilirubin for the screening of neonatal jaundice, it can replace the TSB test to reduce repeated blood sampling. Since this is the first study carried out in term neonates using the TcB test with covering sternum and frontal areas, additional data needs to be collected to verify our findings, so further studies are recommended with higher sample size and using a different type of Bilicheck.
5.1. Strengths and Limitations
One of the major limitations of our study was using one type of Bilicheck instead of two or three versions and also that it was not the newest one. Moreover, sample size was not enough to statistically analyze the difference between extreme premature and premature infants. On the other hand, the main strength of our study is having a new method by covering sternum and frontal that was not applied by other studies.
5.2. Conclusions
The results of this study revealed that the covering of sternal and frontal areas does not affect the correlation between the TcB and TSB methods. Also, the results of this study suggest that TcB in the absence of TSB could be a good alternative in order to measure the level of bilirubin.